* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit 7 Spiraling
Survey
Document related concepts
Transcript
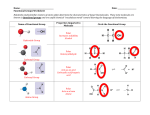
Chemistry: Unit 7 Essential Knowledge Unit 7 Problem Sets (Answers for all problems, additional practice worksheets and a practice test are all eventually posted on Blackboard) Instructions: Do Problems as Assigned. They must be done on a separate sheet of paper in PENCIL. Your full name (first and last), the date, and your class period must be in the upper right corner of your paper. Answers to assigned problems must be legibly printed (no cursive). Typing is only permitted with special permission. SHOWING YOUR WORK IS REQUIRED FOR ALL MATH PROBLEMS!!!!!!!! At the end of every problem set you must write in ink the honor pledge: “On my honor as a W-L student, I have neither given nor received aid on this assignment.” And sign your name. Failure to follow this procedure will result in your paper being returned to you with no credit given. Topic Elements and the Periodic Table 1.7 Essential Knowledge Ionization energy is the energy needed to remove the most loosely held valence electron from a neutral atom. Ionization energy increases going from left to right across a period of the periodic table because the atomic radius decreases, which means that the valence electrons are closer to the nucleus and held more tightly by the nucleus. Ionization energy decreases going down a group because the valence electrons are further away from the nucleus (in a higher energy level) and are more loosely held by the nucleus. Problem Set 1.7 Compounds and Bonding 2.7 1. From your essential knowledge sheet, define ionization energy: 2. From the graph above, which family has the highest ionization energy at energy level? 3. Across a period, ionization energy ______________. 4. Down a family, ionization energy ________________. 5. Circle the element with the greatest ionization energy? F or Ca S or P F or I 6. As the Group 1 elements are considered from bottom to top, the ionization energy of each successive element increases. One reason for this is: a) The positive charge of the nucleus is decreasing b) The number of neutrons is increasing c) The number of principal energy levels is decreasing Distance between the valence electrons and the nucleus is increasing 7. Cesium (Cs) atoms have a) Low electronegativity and high ionization energy b) High electronegativity and low ionization energy c) Low electronegativity and low ionization energy d) High electronegativity and high ionization 8. Ionization energy is the amount of energy needed to _______________ an electron from the outer energy level of an atom. 9. Ionization energy _______________ going down a group (column) because each energy level is _________________ from the nucleus and shielded from the pull of the nucleus by each _______________ energy level below. This causes the electrons to be ______________ attracted to the nucleus and _____________ to remove. 10. Ionization energy _______________ across a period (row) because the atomic radii is getting _____________ so the outer electrons are ________________ to the nucleus. This causes the electrons to be ______________ attracted to the nucleus and _____________ to remove. 11. Arrange the following elements in order of increasing ionization energy. Ar, Na, Si 12. Arrange the following elements in order of increasing ionization energy. Cs, Li, K The shape of a molecule is determined by counting shared and unshared electron pairs in the molecule. Its shape and the polarity of its bonds determine the polarity of a molecule. A linear shape results from two atoms bonded together, or from three atoms bonded together with no unshared electron pairs on the center atom. (N2 and CO2). This shape is nonpolar if the bonds are equivalent but polar if the bonds are different. A tetrahedral shape results from four atoms bonded to a central atom and no unshared electron pairs (CH4). This shape is nonpolar if the bonds are equivalent but polar if the bonds are different. A pyramidal shape results three atoms bonded to a central atom and one unshared electron pair (NH3). This shape is always polar. A bent or angular shape results from two atoms bonded to a central atom and two unshared electron pairs. (H2O and H2S). This shape is always polar. A trigonal planar shape results from three atoms bonded to a central atom and no unshared electron pairs (BH3 and CH2O). This shape is nonpolar if three of the same type of atoms is attached to the central atom, otherwise it is polar. Polarity is determined by the distribution of electronegativity across the molecule. If the distribution is symmetrical (or even), then the molecule is nonpolar. If the distribution is uneven (asymmetrical), then the molecule is polar. Polar molecules have an uneven distribution of electronegativity that causes a dipole (a slight positive Problem Set 2.7 1. Complete the following table Formula Dot Diagram CF4 HF BF3 NF3 Geometry Soluble in H2O? HCOH 2. Shape Identification List the 5 basic shapes we learned in class: 3. Trends a. List the two shapes that are always polar: b. List the three shapes that are symmetrical: c. When are the symmetrical shapes polar? d. When are the symmetrical shapes non polar? 4. Single Bonded Central Atoms: Molecule Shapes a. What shape does a single bonded carbon molecule have? b. What shape does a single bonded oxygen molecule have? c. What shape does a single bonded nitrogen molecule have? d. What shape does a single bonded boron molecule have? e. What shape does a molecule with no central atom have? 5. Sample Questions on Molecular Bonding, Geometry and Polarity a. Which of the following is the correct molecular shape of CH4? A) Bent B) Linear C) Pyramidal D)Tetrahedral b. Elements from which two groups in the periodic table would most likely combine with each other to form an ionic compound? A) 1 and 2 B) 16 and 17 C) 1 and 17 D) 17 and 18 c. In chemical compounds, covalent bonds form when — A) the electronegativity difference between two atoms is very large B) electrons are completely transferred between two metals C) pairs of electrons are shared between two nonmetal atoms _ D) two nonmetal ions are attracted to each other by opposite charges d. Which is nonpolar? a. formaldeheyde b. N2 c. water d. NH3 e. Which of the following is an example of a bent molecule? a. O2 b. N2 c. CO2 d. H2O f. Bonding between two elements of equal electronegativity would be — A) 100% covalent B) primarily ionic C) 50% ionic D) metallic in character g. What shape does the molecule BF3 have? A) Bent B) Linear C) Tetrahedral D) Trigonal planar h. Which formula represents a nonpolar molecule containing polar covalent bonds? (A) H2O (B) NH3 (C) CCl4 (D) H2 i. Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond? (A) metals (B) nonmetals (C) metalloids (D) noble gases j. Based on bond type, which compound has the highest melting point? (A) CH3OH (B) CaCl2 (C) C6H14 (D) CCl4 k. Water is a polar molecule, based on the rules for solubility and the polarity of their molecules, determine which of the following will not be soluble in water. a) HBr b) PF3 c) H2S d) CF 4 l. Which of the following statements is true? a) CO2 is linear, polar and soluble in water b) H2S is linear, nonpolar and soluble in oil c) CH4 is tetrahedral, polar and soluble in oil d) NH3 is pyramidal, polar and soluble in water m. Which of the following is a polar molecule containing three polar covalent bonds and one unbonded electron pairs on the central atom? a) CH4 b) NH3 c) CHF3 d) H2S n. Carbon dioxide is nonpolar because it a. has a pyramidal shape c. has a linear shape b. contains only nonpolar bonds d. has positive ends only 6. The shapes of covalent molecules are determined by comparing the number of ________________ electron pairs to the number of _______________ electron pairs in the Lewis dot diagram. 7. The polarity of a covalent molecule is determined by the distribution of bonds and electron pairs in the molecule. 8. A _________________ molecule has an uneven distribution of electron pairs/bonds around the central atom causing a dipole within the molecule. 9. A _________________ molecule has a even distribution of bonds with no unshared electron pairs around the central atom so no dipole exists. 10. Complete the following table to describe the five most common shapes. 11. The two shapes that are always polar are __________________ and _____________ because they always have unshared pairs around the central atom. 12. For molecules that are linear, trigonal planar or tetrahedral, their polarity depends on the types of bonds. Kinetic Theory 3.7 Molecule # shared pairs # unshared pairs Shape Polarity Cl2 BH3 CH4 NH3 H2O HF HCF3 CO2 13. If all of the bonds are the same, then the molecule is ________________. 14. If the bonds are different, then the molecule is ________________. Pressure and temperature both affect the volume that a gas occupies. Pressure and volume are inversely related; if pressure increases, volume decreases. This is Boyle’s Law Temperature and volume are directly related; if temperature increases, volume increases. This is Charles’ Law Be sure to know Boyles Law, Charles Law, Gay Lussac’s Law, Avogadro’s Law and the combined gas laws. Problem Set 3.7 The Mole and Stoichiometry 4.7 1. On hot days, you may have noticed that potato chip bags seem to “inflate”, even though they have not been opened. If I have a 250 mL bag at a temperature of 19 0C, and I leave it in my car which has a temperature of 600 C, what will the new volume of the bag be? 2. I have made a thermometer which measures temperature by the compressing and expanding of gas in a piston. I have measured that at 1000 C the volume of the piston is 20 L. What is the temperature outside if the piston has a volume of 15 L? What would be appropriate clothing for the weather? 3. Divers get “the bends” if they come up too fast because gas in their blood expands, forming bubbles in their blood. If a diver has 0.05 L of gas in his blood under a pressure of 250 atm, then rises instantaneously to a depth where his blood has a pressure of 50.0 atm, what will the volume of gas in his blood be? Do you think this will harm the diver? 4. A soda bottle is flexible enough that the volume of the bottle can change even without opening it. If you have an empty soda bottle (volume of 2 L) at room temperature (25 0C), what will the new volume be if you put it in your freezer (-4 0C)? 5. Submarines need to be extremely strong to withstand the extremely high pressure of water pushing down on them. An experimental research submarine with a volume of 15,000 liters has an internal pressure of 1.2 atm. If the pressure of the ocean breaks the submarine forming a bubble with a pressure of 250 atm pushing on it, how big will that bubble be? 6. Bolyes Law states that pressure and volume are________________ related. 7. Charles’ Law states the temperature and volume are ________________ related. 8. At constant temperature, if the pressure decreases the volume will _______________. 9. At constant pressure, if the temperature decreases the volume will _______________. 10. At constant temperature, if the pressure is tripled then the volume will be _______________. 11. At constant pressure, if the volume is halved then the temperature will be _______________. 12. Sketch the graph of temperature verses volume. 13. Sketch the graph of pressure verses volume. The number of moles of a gas (n) can be determined if the pressure (P), temperature (T) and volume (V) of the gas sample are known, using the constant R according to the Ideal Gas Law equation: PV = nRT When using the Ideal Gas Equation, temperature must be in Kelvin, and all other units must match the units of the gas constant R. Values of R will always be given to you, but they must match the units of the other numbers in the equation. R = 8.314 L kPa/mol K or 0.0821 L atm/ mol K Problem Set 4.1 1. If I have 4 moles of a gas at a pressure of 5.6 atm and a volume of 12 liters, what is the temperature? 2. If I have an unknown quantity of gas at a pressure of 1.2 atm, a volume of 31 liters, and a temperature of 87 0C, how many moles of gas do I have? 3. If I contain 3 moles of gas in a container with a volume of 60 liters and at a temperature of 400 K, what is the pressure inside the container? 4. If I have 7.7 moles of gas at a pressure of 0.09 atm and at a temperature of 56 0C, what is the volume of the container that the gas is in? 5. If I have 17 moles of gas at a temperature of 67 0C, and a volume of 88.89 liters, what is the pressure of the Chemical Reactions 5.7 gas? 6. If I have an unknown quantity of gas at a pressure of 0.5 atm, a volume of 25 liters, and a temperature of 300 K, how many moles of gas do I have? 7. If I have 21 moles of gas held at a pressure of 78 atm and a temperature of 900 K, what is the volume of the gas? 8. Complete the following table related to the Ideal Gas Law PV=nRT symbo represents units l pressure P L or dm3 V n Ideal Gas L atm/moles K; dm3 atm/moles K; L mmHg/moles K; dm3 R Constant mmHg/molesK; L kPa/moles K or dm3 kPa/moles K T 9. Find the number of moles of carbon dioxide if it occupies 5.0 L at 1.2 atm and 30.0 oC. Show work! (R= 0.0821) 10. What volume would this sample of carbon dioxide occupy at STP? Show work! 11. How many moles of helium gas would there be under the same conditions? _____________ Predict products of single and double replacement reactions. *Note that nitrate (NO3)1- is a polyatomic ion that acts as a single particle with a single charge. Also, know the charges for the sulfate (SO4 )2- ion, ammonium ion (NH4 )1+, hydroxide ion (OH )1-, carbonate ion (CO3 )2-, and the phosphate ion (PO4 )3-. Combustion reactions are exothermic reactions in which oxygen combines with other elements producing carbon dioxide and water. One example is the reaction between methane and oxygen in a Bunsen burner: CH4(g) + 2O2(g) CO2(g) + 2H2O(g) + energy Problem Set 5.7 Solutions 6.7 Balance the equations and predict the products for the following reactions: 1) ____ Na + ____ FeBr3 2) ____ C2H4O2 + ____ O2 3) ____ PbSO4 + ____ AgNO3 4) ____ PBr3 5) ____ Mg Br2 + ____ Li 6) ____ KMnO4 + ____ ZnCl2 7) ____ O2 + ____ C5H12O2 Write the formula for each compound: 1. ammonium sulfate ____________________ 2. sodium phosphate ____________________ 3. Iron (III) oxide _____________________ 4. methane ___________________________ 5. Lead (II) hydroxide _____________________ 6. Copper (I) chloride ___________________ 7. Magnesium nitrate ______________________ 8. carbon dioxide ______________________ 9. ammonia ______________________________ 10. Aluminum carbonate __________________ 11. Single replacement reactions can be identified because an ______________ + a____________________ produce a different ________________ + _______________. 12. Double replacement reactions can be identified because the reactants are both ________________ and the products are two new ____________________. 13. Combustion reactions always have ________________ as a reactant and produce _________________ + _________________ as products. 14. Classify each of the following unbalanced reactions: a. C2H6 + O2 CO2 + H2O ___________________________ b. CO2 + H2S CS2 + H2O ___________________________ c. Cl2 + CH4 CCl4 + H2 ___________________________ d. Ag + HNO3 AgNO3 + H2 ___________________________ The Solubility of a substance represents the maximum amount (in grams) of that substance that can dissolve in 100 grams of water at 250C and is usually expressed as grams of solute/100 grams of water. When a solution holds the maximum amount of solute it is considered to be saturated and has reached the solubility point for that substance. The dissolving process is reversible. A saturated solution is at equilibrium when the rate of dissolving is equal to the rate of re-crystallization. The relative solubility of a solute (whether something will dissolve or not) is summarized by the phrase “like dissolves like”. Solutes will dissolve in solvents with similar intermolecular attractions. Problem Set 6.7 Experimentation 7.7 1. What happens to the solubility of most solids as the temperature increases? 2. What happens to the solubility of gases as the temperature increases? 3. Using the curve, what is the solubility of NaNO3 at 40oC? 4. If you add 20 grams of KNO3 to water at 30oC is the solution saturated or unsaturated? 5. If I add 70 grams of NH4Cl to water at 700C, how much solid will be left undissolved on the bottom of the beaker? 6. How much KClO3 can dissolve in 250 grams of water at 20oC? 7. The solubility of a substance represents the mass of __________________ that can dissolve in 100 g of ____________________ at a given temperature. 8. Generally, as the temperature of the water increases, the solubility of solids ______________ and the solubility of gases _________________. 9. If 120g of solid substance X dissolves in 50 g of water at 50.0 oC, what is its solubility at 50.0oC? Show work! 10. Would the solubility increase or decrease if the solution were cooled to 25.0 oC? ___________ 11. If 2.5 grams of gaseous CO2 dissolves in 1000 grams of water at 10.0oC, what is its solubility at 10.0oC? Show work! Pressure is a measure of the number and force of the collisions of gas particles. 1 atm = 760 mmHg = 101.3 kPa Volume measures the space occupied by the gas particles. 1 liter = 1000 mL = 1000 cm3 = 1 dm3 Temperature is the measure of the average kinetic energy of the gas particles. This must be expressed in Kelvin (K) when using the gas laws. 273K = 0 o C Problem Set 7.7 1. 2. Unit conversions for measuring pressure, temperature and volume are important to know when using gas laws. a. 1 atm = ______ mmHg = _______ kPa b. 0.0oC = ______ K c. 1.0 L = ________ dm3 1.0 mole of any gas at STP occupies 22.4 liters. Indicate the correct value for each of the following: a. volume in mL = ___________ b. pressure in atm = ____________ c. temperature in K = ____________ d. pressure in kPa = ______________ e. temperature in oC = ____________ Mastery Knowledge – Unit 7 1 2 3 4 5 Gases have mass and occupy space. Gas particles are in constant, rapid, random motion and exert pressure as they collide with the walls of their containers. Equal volumes of gases at the same temperature and pressure contain an equal number of particles. An ideal gas is one whose particles exhibit absolutely no attractive or repulsive forces, and the volume of each particle is assumed to be insignificant. An ideal gas does not exist, but this concept is used to model gas behavior. Real gases have intermolecular forces, particle volume, and can change states. (Review intermolecular forces on Unit 6 Extensions.) Intermolecular forces determine many physical properties of a substance, like physical state, vapor pressure and solubility. Molecular substances with weak intermolecular attractions are gases at room temperature, while those with stronger attractions between particles are liquids or solids at room temperature. The stronger the attractive forces between the particles, the greater the heat of fusion A liquid in a closed container will evaporate until the air in the container is saturated with vapor, and a dynamic equilibrium exists between the gas and the liquid. At equilibrium, the particles of a substance will 6 7 8 continue to evaporate and condense, but there is no overall change in the number of either liquid or vapor particles. The pressure exerted by the gaseous particles of a substance in a closed container is the vapor pressure. Equilibrium vapor pressure is directly related to the temperature of the substance and indirectly related to its attractive forces. The weaker the attractive forces between the particles, the higher the vapor pressure. Specific Heat Capacity is the amount of heat needed to raise 1 g of a substance 1ºC. The heat capacity of water is 4.18J/g·ºC. Water and other insulators have high heat capacities therefore, it is harder to the raise their temperature and they retain heat longer. Metals and other conductors have low heat capacities therefore, it is easier to raise their temperature and they lose heat faster. Because of water’s high heat capacity and low toxicity it is the preferred liquid used in calorimetry experiments. Molar heat of fusion (Hfus) is the amount of energy absorbed when one mole of a substance to melts. The heat lost when one mole of a liquid solidifies is the molar heat of solidification (Hsolid). The amount of heat absorbed by a melting solid is exactly the same as the amount of heat lost when the liquid solidifies, so Hfus = -Hsolid. When liquids absorb heat at their boiling points, the become vapors. The amount of energy needed to vaporize one mole of a given substance is called its molar heat of vaporization (Hvap). Condensation is the exact opposite of vaporization. The amount of heat released when 1 mol of vapor condenses is called its molar heat of condensation (Hcond). Thus, Hvap = -Hcond. Problem Set for Mastery Knowledge Define Temperature, Heat, Exothermic, Endothermic What is the first law of thermodynamics? What are two units for measuring heat and how are they defined? What is specific heat capacity? What is the specific heat capacity of water? What equation do you use when you are figuring the amount of heat required to raise or lower the temperature of a substance? What is a calorimeter? Practice Problems: 1. How much heat is needed to raise the temperature of 500.0 grams of water 2.00 degrees Celsius? Express your answer in both calories and joules. 2. Calculate the amount of heat needed to bring one liter of water (density of water is 1g/ml) from room temperature (25oC) to the boiling point. Express your answer in both calories and joules. 3. Hoe much heat is absorbed by an aluminum cookie sheet with a mass of 50.0 grams as it heats from 25 OC to 100OC? (The specific heat capacity of aluminum is 0.89 J/gOC) Express your answer in both calories and joules. 4. What is the specific heat capacity for aluminum if the temperature of a 28.4 g sample of aluminum rises by 8.1OC when 207 J of heat are added? 5. Read pp.446-448 and then define Molar Heat of Vaporizaton: Molar Heat of Fusion: Draw a heating and cooling curve for water (p.445) Show all the steps in the self-check 14.1 on p. 448.