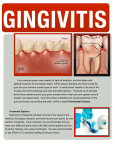
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Trabajo Práctico 1
Survey
Document related concepts
Transcript
Trabajo Práctico No 1 Farmacología de los quelantes 1- Artículo: Casarett & Doull’s Toxicology 2- Defina: a) Quelante b) ¿Es un antídoto? ¿O un antagonista? ¿Que diferencias-similitudes encuentra con éstos? c) Organice en columnas a los siguientes quelantes – antagonistas - antídotos según corresponda: Atropina (OP), BAL, Flumazenil (Benzodiacepinas), Quelocianor, EDTA, Naloxona (opiáceos), Pralidoxima (OP), Fracc FAB, Protamina, D penicilamina, Deferroxamina Quelante Antídoto Antagonista d) Complete mencionando al menos tres efectos adversos de cada uno, contraindicaciones, y vías de administración. QUELANTES VA EFECTOS ADVERSOS PrecaucionesContraindicaciones EDTA Ca Na2 DIMERCAPROL (BAL) D-PENICILAMINA e) Una con flechas. BAL Deferroxamina D penicilamina EDTA Quelocianor Intox aguda plomo (primera elección) Intox aguda plomo (segunda elección) Hg inorgánico Hg orgánico Cobre Arsénico agudo y crónico Cianuro Hierro 1 Cat. Embarazo Casos clínicos- Medidas de rescate 1- Paciente de 30 años. Glasgow 8/15. Al exámen físico presenta miosis e hipotonía de 2 horas de evolución. Al interrogatorio la esposa refiere que el paciente recibe Alprazolam pues en los últimos meses se encontraba ¨muy nervioso¨. a) ¿Cuál es su sospecha diagnóstica? b) ¿Cuál es la primera medida que se debe tomar teniendo en cuenta el score de Glasgow del paciente? c) ¿Tiene indicación de LG este paciente? ¿Cómo se realiza? ¿Y de VP? d) ¿Se podría administrar algún antídoto/antagonista/quelante? Discuta con sus compañeros. 2- Paciente de 35 años que se encuentra desorientado. Presenta excitación psicomotriz, se encuentra febril (T°39°), e hipotenso. a) ¿Qué es la Imipramina? b) ¿La sobredosis de ésta explica los síntomas? ¿Sería útil solicitar un ECG? c) Conociendo el tiempo de evolución del cuadro, ¿se podría indicar LG, VP, CA? ¿Cómo tendría que administrarse en este caso? d) ¿Conocé otra medida de rescate que se pueda utilizar en este caso? COMPLEXATION AND CHELATION THERAPY Treatment of poisoning from toxic metals is sometimes warranted to prevent or reverse toxicity and therefore remains an important topic, particularly for those metals that are cumulative and persistent (e.g., lead). It must be emphasized, however, that treatment is only a secondary alternative to reduction or prevention of exposures to toxic metals. Complexation is the formation of a metal ion complex in which the metal ion is associated with a charged or uncharged electron donor, referred to as a ligand. The ligand may be monodentate, bidentate, or multidentate; that is, it may attach or coordinate using one, two or more donor atoms. Chelation occurs when bidentate ligands form ring structures (chelate comes from the Greek word for claw) that include the metal ion and the two ligand atoms attached to the metal (Williams and Halstead, 1982). Metals may react with O-, S-, and N-containing ligands present in the form of OH, COOH, SH, NH2, NH, and N. A resultant metal complex is formed by coordinate bonds (coordination compound), in which both electrons are contributed by the ligand (Klaassen, 1990). Chelating1 agents (drugs) vary in their specificity for toxic metals. Ideal chelating agents should be water-soluble, resistant to biotransformation, able to reach sites of metal storage, capable of forming nontoxic complexes with toxic metals, and of being excreted from the body; they should also have a low affinity for essential metals, particularly calcium and zinc (Klaassen, 1990). The challenge in the development of safer and more effective chelating agents is to design all of the desirable chemical, physiologic, and pharmacologic properties into the drug (Jones, 1992). The general properties of chelating agents that are of current interest are briefly described below. Additional details and comments are provided later in the chapter with discussions of specific metals. BAL (British Anti-Lewisite) BAL (2,3-dimercaptopropanol) was the first clinically useful chelating agent. It was developed during World War II as a specific antagonist to vesicant arsenical war gases, based on the observation that arsenic has an affinity for sulfhydryl-containing substances. BAL, a dithiol compound with two sulfur 2 atoms on adjacent carbon atoms, competes with the critical binding sites responsible for the toxic effects. These observations led to the prediction that the “biochemical lesion” of arsenic poisoning would prove to be a thiol with sulfhydryl groups separated by one or more intervening carbon atoms. This prediction was borne out a few years later with the discovery that arsenic interferes with the function of 6,8-dithiooctanoic acid in biological oxidation (Gunsalus, 1953). BAL has been found to form stable chelates in vivo with many toxic metals, including inorganic mercury, antimony, bismuth, cadmium, chromium, cobalt, gold, and nickel. However, it is not necessarily the treatment of choice for toxicity to these metals. BAL has been used as an adjunct in the treatment of the acute encephalopathy of lead toxicity. It is a potentially toxic drug, and its use may be accompanied by multiple side effects. Although treatment with BAL will increase the excretion of cadmium, there is a concomitant increase in renal cadmium concentration, so that its use in case of cadmium toxicity is to be avoided. It does, however, remove inorganic mercury from the kidney, but it is not useful in the treatment of alkyl mercury or phenyl mercury toxicity. BAL also enhances the toxicity of selenium and tellurium, so it is not to be used to remove these metals from the body. DMPS DMPS (2,3-dimercapto-1-propanesulfonic acid) is a water-soluble derivative of BAL developed in response to BAL's toxicity and unpleasant side effects. DMPS has been shown to reduce blood lead levels in children (Chisholm and Thomas, 1985). It has the advantage over ethylene diamine tetraacetic acid (EDTA) in that it is administered orally and does not appear to have toxic side effects. It has been widely used in the former Soviet Union to treat many different metal intoxications. DMPS has been used experimentally to estimate the renal burden of lead (Twarog and Cherian, 1984) and inorganic mercury (Cherian et al., 1988). Its effectiveness in mobilizing metals from the kidney may be due to the fact that it is transported into kidney cells on the organic anion transport system (Zalups et al., 1998). It increases the urinary excretion of mercury in persons with an increased body burden from industrial exposure, from dentists and dental technicians, from persons with dental amalgams, from those exposed to mercurous chloride in skin creams (Aposhian et al., 1992; Gonzalez-Ramirez et al., 1998). This agent has also been used to assess body burdens of inorganic mercury from dental amalgams and of arsenic ingested from drinking water (Aposhian, 1998). DMSA DMSA (meso-2.3.-dimercaptosuccinic acid; succimer), like DMPS, is a chemical analog of BAL. More than 90 percent of DMSA is in the form of a mixed disulfide in which each of the sulfur atoms is in disulfide linkage with a cysteine molecule (Aposhian and Aposhian, 1992). The drug is of current interest clinically because of its ability to lower blood lead levels. It has advantages over EDTA because it is given orally and has greater specificity for lead. It may be safer than EDTA in that it does not enhance excretion of calcium and zinc to the same degree. Studies in rodents showed that a single dose of DMSA primarily removes lead from soft tissues (Smith and Flegal, 1992). A lead mobilzation test with DMSA does not appear to give better information on body burden than measurements of lead in blood, plasma, or urine (Gerhardsson et al., 1999). The drug has been licensed recently by the U.S. Food and Drug Administration (FDA) specifically for the treatment of lead poisoning in children whose blood lead levels are ³45 µg/dL, and it has been used in Europe. However its effectiveness in improving long-term blood lead levels in children has been questioned (O'Connor and Rich, 1999), nor, according to studies on primates, does it appear effective in removing lead from the brain (Cremin et al., 1999). Its ability to reverse some of the toxic outcomes of lead poisoning, such as negative effects on cognitive and behavioral development, has not been demonstrated. EDTA Calcium salt of ethylene diamine tetraacetic acid (EDTA) must be used for clinical purposes because the sodium salt has greater affinity for calcium and will produce hypocalcemic tetany. However, the calcium salt will bind lead, with displacement of calcium from the chelate. EDTA is poorly absorbed from the gastrointestinal tract so it must be given parenterally, which distributes it rapidly throughout the body. It has long been the method of choice for the treatment of lead toxicity. The peak excretion point is within the first 24 h and represents the excretion of lead from soft tissues. Removal of lead from the skeletal system occurs more slowly, with the restoration of equilibrium with soft tissue compartments. Animal experiments indicate that EDTA is not effective in 3 reducing total brain lead (Seaton et al., 1999). Calcium EDTA does have the potential for nephrotoxicity, so it should be administered only when clinically indicated (EPA, 1986). Combination therapy of EDTA with other chelating agents, such as BAL and DMSA, has been used to reduce the risk of side effects from either agent alone, as each agent can be given at a lower dose in combined therapy. Whether BAL or DMSA is used as the second agent, the results are equally effective in reducing blood lead in children (Besunder et al., 1997). DTPA DTPA, or diethylenetriaminepentaacetic acid, has chelating properties similar to those of EDTA. The calcium salt (CaNa2DTPA) must be used clinically because of DTPA's high affinity for calcium. It has been used for the chelation of plutonium and other actinide elements, but with mixed success. Experimental studies have shown that various multidentate hydroxypyridinonate ligands are more effective than CaNa2DTPA for promoting excretion of Pu and other actinides (Durbin et al., 2000). Desferrioxamine Desferrioxamine is a hydroxylamine isolated as the iron chelate of Streptomyces pilosus and is used clinically in the metal-free form (Keberle, 1964). It has a remarkable affinity for ferric iron and a low affinity for calcium, and it competes effectively for iron in ferritin and hemosiderin but not in transferrin and not for the iron in hemoglobin or heme-containing enzymes. It is poorly absorbed from the gastrointestinal tract, so it must be given parenterally. Clinical usefulness is limited by a variety of toxic effects, including hypotension, skin rashes, and possibly cataract formation. It seems to be more effective in hemosiderosis due to blood transfusion but is less effective in treatment of hemochromatosis. A high-molecular-weight derivative in which desferrioxamine is chemically coupled to hydroxyethyl starch shows promise in human tests as an effective iron chelator, with lower toxicity than the parent compound (Dragsten et al., 2000). Dithiocarbamate (DTC) Dithiocarb (diethyldithiocarbamate), or DTC, has been recommended as the drug of choice in the treatment of acute nickel carbonyl poisoning. The drug may be administered orally for mild toxicity and parenterally for acute or severe poisoning (Sunderman, 1979). However, no adequately controlled clinical studies have been performed (Bradberry and Vale, 1999). DTC has also been used experimentally for removal of cadmium bound to metallothionein (Kojima et al., 1990). A number of DTC compounds with various substitutions of nonpolar, nonionizing groups have been synthesized by Jones and Cherian (1990). Sodium, N-(4-methoxybenzyl)-Dglucamine dithiocarbamate (MeOBGDTC), is among the most effective in removing cadmium from tissues. The CdMeOBGDTC complex is excreted in the bile rather than by the kidney, avoiding the nephrotoxicity characteristic of cadmium chelates. To date the use of this compound has been limited to experimental studies in rodents. Penicillamine and N-Acetylcysteine Penicillamine (b, b-dimethylcysteine), a hydrolytic product of penicillin, has been used for the removal of copper in persons with Wilson's disease and for the removal of lead, mercury, and iron (Walshe, 1964). It is also important to note that penicillamine removes other physiologically essential metals, including zinc, cobalt, and manganese. Attached to its use is the risk of inducing a hypersensitivity reaction with a wide spectrum of undesirable immunologic effects, including skin rash, blood dyscrasia, and possibly proteinuria and nephrotic syndrome. It has cross-sensitivity with penicillin, so it should be avoided by persons with penicillin hypersensitivity. For persons who have developed a sensitivity to penicillamine, an orally active chelating agent, triethylene tetramine 2HCl (Trien), is an alternative for the removal of copper (Walshe, 1983). Reducing the commonly used dose from 25 to 30 mg/kg/day to 15 mg/kg/day maintains the efficacy of d-penicillamine at reducing blood lead with reduced side effects in lead-exposed children (Shannon and Townsend, 2000). N-acetylcysteine has been widely used clinically as a mucolytic agent (e.g., for cystic fibrosis) and to protect against the toxic effects of a number of chemicals. It is a free-radical scavenger, a precursor to glutathione, and it can form stable water-soluble complexes with mercury and other metals. Its ease of administration (oral), low toxicity, and wide availability in the clinical setting makes it an attractive therapeutic agent. It is effective in accelerating the removal of methyl mercury in animal tests (Ornaghi et al., 1993; Ballatori et al., 1998). It was also effective in extracorporeal complexation hemodialysis (see below) in one case of human exposure to methyl mercury (Lund et al., 1984). 4 Hemodialysis with Chelation Hemodialysis is usually not effective in removing metals from the bloodstream because many metals are associated with red blood cells and/or bound to plasma proteins (for example, Sauder et al., 1988). However a chelating agent may transform the metal into a diffusible form amenable to removal by hemodialysis (Kostyniak and Clarkson, 1981). The chelating agent may be given systemically, as in the application of desferrioxamine to remove aluminum in conjunction with hemodialysis (Nakamura et al., 2000). Extracorporeal complexation hemodialysis avoids systemic application of the complexing agent by introducing the agent into the blood as it enters the dialyzer. Hemodialysis then removes the metal chelate and as well as the excess chelating agent. The method was first successfully used to remove methyl mercury from intoxicated patients (Al-Abbasi et al., 1978), and it has subsequently been applied to a case of inorganic poisoning (Kostyniak et al., 1990). The most effective complexing agent appears to be DMSA for mercury removal with hemodialysis (Kostyniak, 1982). The method has been successfully tested in dogs to remove cadmium using a combination of EDTA and glutathione as complexing agents (Sheabar et al., 1989). Trabajo Práctico No 2 1- Defina y de un ejemplo de los siguientes enunciados: a) Hipoxia hipóxica b) Hipoxia anémica c) Hipoxia histotóxica 2- Relacionar con flechas salamandras calefón estufa fertilizantes monóxido de carbono propelentes cianuro acrilico incendios hidrocarburos gaseosos gas natural laurel carozo de duraznos 3- Usted se encuentra de guardia en un Htal periférico y llega Maria que tiene 30 años y esta embarazada de 7 meses, concurre con José de 2 años y Carola de 12 meses de edad. Le comenta que hoy después de bañar a José y Carola ella empezó a tener un fuerte dolor de cabeza y ganas de vomitar pero se preocupo más al ver que José estaba chinchudo, decaído, medio molesto y con mucho sueño. En cambio Carola no quiso comer. Responda: a) Usted sospecha que se intoxicaron con: 5 b) ¿Qué preguntas pueden ser importante que usted le haga para determinar el tipo de intoxicación que tiene? c) ¿Qué estudios usted le pediría? d) ¿Cómo se debería encontrar el color de la piel? e) ¿Es relevante que esté embarazada? ¿Por qué? f) ¿Qué tratamiento realizaría? 4- Ante un paciente inconsciente, con síntomas de asfixia, con la piel sonrosada a la que se extrae sangre de color achocolatado, la causa más probable de intoxicación es por: A. propano butano B. Cianuro. C. Monóxido de carbono. D. Gas arsina E. Todas son correctas 5- Señalar cuál de las siguientes afirmaciones es falsa en relación con la intoxicación por monóxido de carbono: A. Es un gas con 200 veces mayor afinidad por la hemoglobina que el oxígeno. B. Desplaza la curva de disociación de la hemoglobina a la izquierda. C. Es el principal responsable de la intoxicación por humo en incendios. D. La tos e irritación de la vía aérea superior es característica de las formas leves de intoxicación. E. El tratamiento se fundamenta en la administración de oxígeno a elevadas concentraciones. 6- La toxicidad hepática del tetracloruro A. Es producida por metabolitos activos B. Es producida por radicales libres C. Se presenta en las intoxicaciones cronicas D. Ninguna es correcta 7- El cianuro se caracteriza por A. su alta toxicidad que lleva a cuadros letales B. Produce una gran cantidad de intoxicados todos los años C. Bloquear la respiración celular D. Todas son correctas E. A y C son correctas 8- En cuanto a los hidrocarburos A. Se los puede dividir en dos grupos saturados y no saturados B. El propano y butano se ubican en el grupo de los hidrocarburos no saturados C. El tetracloruro de carbono es un derivado del propano D. El tratamiento para la intoxicación por hidrocarburos es Vomito provocado, carbón activado y purgante salino mas el tratamiento sintomático adecuado 9- Con respecto al cianuro A. su alta afinidad por el hierro en estado ferroso es lo que lo une a las enzimas de la cadena respiratoria. B. La intoxicación por altas dosis lleva a la muerte en forma inmediata. C. El tratamiento especifico es la utilización de hidroxicobalamina por su rápida unión al cianuro y 6 eliminación por orina. D. Ninguna es correcta. 10- Escriba en la linea punteada 5 indicaciones de camara hiperbárica: 1………………………. 2………………………. 3………………………. 4………………………. 5………………………. 11- ¿Qué secuelas se pueden presentar con monóxido de carbono y que complicaciones con propano y butano? 12- Con lo ya visto ahora puede completar el siguiente cuadro: MONOXIDO DE CARBONO CIANURO PROPANO Y BUTANO HIPOXIA DIAGNOSTICO TRATAMIENTO Trabajo Práctico No 3 Hidrocarburos 1- Describa las 3 vias principales por las que puede producirse un cuadro de intoxicación por HC. ¿Cúal le parece que es la más frecuente? 2- ¿Cúal es el mecanismo de acción de los HC? ¿Le recuerda a algún otro cuadro clinico? 3- ¿Cómo suele presentarse estos pacientes? ¿Cúal suele ser una complicación habitual? 4- ¿Qué estudios complementarios solicitaría y que esperaría encontrar? 5- ¿Qué conductas NO adoptaría con estos pacientes? 6- Responda con Verdadero o Falso con respecto a los Hidrocarburos: 7 a) El Benzol es un compuesto muy toxico por que se forma de la combinación del Benceno + Tolueno + Xileno. b) El Benceno se manifiesta en forma crónica con Alteraciones en el SNC. c) El tetracloruro de carbono produce radicales libres que lesionan el hígado. d) El tratamiento de decontaminacion de los hidrocarburos es Vomito provocado carbón activado y purgante salino. e) El sme neuropsiquiatrico con ataxia cerebelosa se presenta en personas que trabajan habitualmente con tolueno y en personas que inhalan pegamento. Tetracloruro de Carbono 1- ¿Dónde podemos encontrar al TTC? Hasta no hace mucho, el TTC formaba parte de algunos compuestos utilizados en Medicina, ¿sabe cuales? 2- ¿Cúal es su mecanismo de acción? ¿Conoce alguna sustancia y/o fármaco que pueda potenciar su acción? ¿Por qué? 3- ¿Qué cuadro clinico espera encontrar en un paciente que cursa una intoxicación aguda por TTC? ¿Y en una intoxicación crónica? 4- ¿Qué estudios de laboratorio NO dejaría de solicitar a este paciente? 5- ¿Cómo lo trataría? ¿Su conducta sería diferente ante este paciente vs un paciente intoxicado por otro HC? ¿Utilizaria N-Acetilcisteina? Justifique. Tolueno 1- Cuando piensa en Tolueno, ¿cúal es el perfil de paciente en el que debería sospechar su abuso? ¿Sólo en esos pacientes? ¿Existen pacientes que no abusen de esta sustancia pero que puedan intoxicarse de todas maneras? 2- Describa su mecanismo de acción y metabolismo. 3- ¿Cómo es el cuadro clínico? Explayesé. ¿Cómo espera encontrar el estado ácido-base? ¿A qué se debe? 4- Al conocer el metabolismo del Tolueno, ¿se le ocurre alguna manera de confirmar su sospecha? ¿Pueden existir falsos positivos? ¿Qué valores consideraría patológicos? ¿En cualquier paciente? 5- ¿Cómo piensa que es el tratamiento de la intoxicación por Tolueno? Metahemoglobinemia 1- Defina Metahemoglobina. 2- La sola presencia de MetaHb, ¿nos está hablando de un estado patológico? Justifique su respuesta. 3- Piense y discuta con sus compañeros las posibles causas de elevación de los valores de MetaHb. 8 4- ¿Qué tipo de pacientes corren mayor riesgo de tener valores patológicos de MetaHb? 5- Describa el cuadro clínico. ¿Qúe debería tener en cuenta? 6- ¿Cuál es el tratamiento espécifico de este cuadro? ¿Sabe de alguno más? Especifique en que casos los utilizaría. MULTIPLE CHOICE: 1- Con respecto a la anilina ¿cuál de las siguientes afirmaciones es falsa?: A. Es una sustancia utilizada en la industria de los tintes B. Produce hemólisis C. Es un agente metahemoglobinizante D. Produce anemia aplásica E. Se absorbe a través de la piel 2- Cuál de las siguientes causas NO es una fuente de exposición a agentes metahemoglobinizantes: A. Ingesta de agua de pozo B. Anestésicos locales C. Administración de antileprosos D. Uso de antisépticos E. Uso de aguarrás 3- De las siguientes afirmaciones, señalar cuál de ellas es FALSA A. En la metahemoglobina el hierro se encuentra en estado férrico B. La metahemoglobina se produce por oxidación de la hemoglobina C. El permanganato potásico, utilizado como antiséptico, produce metahemoglobinemia D. En la metahemoglobinemia la SO2 por pulsioximetría es baja E. La cianosis, en la metahemogloinemia, tiene un color azul pizarroso característico. 4- ¿Cuál es la vía de entrada de los tóxicos metahemoglobinizantes? A. Inhalatoria B. Cutánea C. Oral D. Sólo a) y c) son ciertas E. Todas las anteriores son ciertas 5. ¿Cuál de los siguientes signos y/o síntomas NO aparecen en la metahemoglobinemia aguda tóxica? A. Rabdomiolisis B. Cianosis C. Pérdida de conciencia D. Cefaleas E. Taquicardia 9 6. La intoxicación por tolueno: A. Es frecuente en niños-adolescentes que inhalan colas. B. Es neurotóxico. C. Puede provocar muerte súbita por afectación cardíaca. D Todas las anteriores son ciertas. E. Ninguna de las anteriores es cierta. 7. El antídoto de elección en la intoxicación por anilinas es: A. Acetilcisteina. B. Fisostigmina. C. Azul de metileno. D. Flumacenilo. E. No existe antídoto. 8. En la intoxicación por tetracloruro de carbono la hepatitis podría tratarse con: (fundamente su respuesta) A. Acetilcisteina. B. Fisostigmina. C. Azul de metileno. D. Flumacenilo. E. Ninguno de los anteriores. 9. La presencia de metahemoglobinemia sugiere la intoxicación por uno de los siguientes disolventes: A. Tolueno. B. Benceno. C. Cloroformo. D. Anilina. E. Ninguno de los anteriores. 10. La manifestación clínica más característica de la intoxicación crónica por benceno es: A. Hepatitis. B. Insuficiencia renal. C. Neuropatía periférica. D. Miocardiopatía. E. Anemia aplásica. Trabajo Práctico No 4 Caso Clínico – Etanol Metanol El miércoles pasado, a la 1 de la mañana llegaron 3 jóvenes acompañados de 2 amigos a su guardia. Cuando usted se acerca a ellos para atenderlos se da cuenta que los 3 se encontraban con un claro cuadro de “embriaguez”, presentaban incoordinación motora, disartria y náuseas. 10 Además dos de ellos tenían un fuerte aliento alcohólico. Este cuadro le suena familiar y usted inmediatamente piensa en una intoxicación alcohólica. 1-¿Qué otros diagnósticos presuntivos se le ocurren? 2-¿Qué medida tomaría inicialmente? Durante el interrogatorio los amigos que trajeron a los chicos le cuentan que era su fiesta de egresados y por ese motivo habían alquilado el “Trencito de la Alegría” para que los transporte hasta el salón. Mientras estaban en el tren consumieron diferentes tipos de alcohol, cerveza, vodka, ron, licor de menta y fernet. Ambos niegan que alguno de sus compañeros hayan consumido algún tipo de droga junto con el alcohol. 3-¿Cuál cree que es el siguiente paso a seguir? 4-¿Cuándo en los pacientes con intoxicación etílica debe realizarse hemodiálisis? Al mismo tiempo que llegan los resultados de laboratorio, uno de los pacientes comienza a quejarse de que ve “algo borroso” y al revisar los exámenes de laboratorio comprueba que el análisis de gases en sangre de este paciente muestra una marcada acidosis en comparación con los resultados de sus amigos. 5-¿Qué cree que paso con este paciente y que medidas tomaría? 6- ¿Cuál seria el tto para este paciente? 7- ¿Cuáles serian las indicaciones de hemodiálisis en estos pacientes? Gracias a su eficaz tto, los pacientes mejoran, sin embargo ud cree que debe hacer algo más por estos chicos….¿que podría ser? Caso Clínico - Intoxicación por Etilenglicol. Ud, que es el Jefe de Residentes de Toxicología del Hospital Fernández, estaba durmiendo plácidamente, cuando es despertado por un residente de segundo año de Clínica desesperado porque recibió un llamado donde le avisaban que estaban transportando en ambulancia a una mujer que había intentado suicidarse al ingerir etilenglicol. José, el residente, esta desesperado porque hace 4 años que cursó toxicología y no se acuerda absolutamente nada. Ud, se levanta y mientras espera la ambulancia le refresca a José cuales son las posibles fuentes del etilenglicol. 1-¿Cuáles son las posibles fuentes de etilenglicol? Al llegar la ambulancia ven que Sara de 61 años, se encontraba estuporosa pero conciente. Proceden a hacerle el examen físico básico que resultan en tensión arterial de 120/70 mmHg, frecuencia cardíaca de 110 x’ y frecuencia respiratoria de 30 x’. La paciente se encuentra letárgica, con capacidad para mover los 4 miembros y seguir órdenes simples. Ud inmediatamente indica que le realicen un análisis de gases en sangre, estado ácido base, un análisis de función renal, un análisis de orina y dosaje de etilenglicol en sangre. José, totalmente desconcertado porque los signos y síntomas que presenta Sara se parecen mucho a una intoxicación por etanol y no encuentra explicación para el análisis de función renal que ud pidió. 2-¿Cuál es la utilidad de los exámenes solicitados? Mientras ud le explicaba a José comenzó a realizarle lavado gástrico a la paciente. 11 3-¿Es de utilidad realizarle medidas de rescate en este tipo de intoxicaciones? Mientras realizaba el lavado gástrico le pidió a la enfermera que buscara ampollas de tiamina y piridoxina y como vió que el residente no se animaba a preguntarle, ud respiro profundo y directamente le explicó…. 4-¿Cuál es la utilidad de las vitaminas en el tratamiento? Los resultados del laboratorio informan Na+ 144 mmol/L, Bicarbonato 13 mmol/L, urea plasmática 15 mg/dl, Creatinina de 0,9 mg/dl y Glucosa de 147 mg/dl. Calcemia disminuida. El anión gap 25 mmol/L. Ph arterial de 7,17, PCO2 de 28 mmHg y HCO3 de 11 mmol/L. La osmolaridad plasmática es de 365 mOsm/Kg. En orina se encontró cristales de oxalato de calcio y los niveles de Etilenglicol en sangre son de 202 mg/dl. Dados los resultados de laboratorio ud le pregunta a José cual cree que es el mejor tratamiento inicial y el rápidamente responde etilterapia.!!! Esta respuesta si bien a simple vista parece correcta, esta mal……. 5- ¿Por qué? Etanol Metanol Fuentes Cinética Clínica Diagnositico Tratamiento Trabajo Práctico No 5 1- En relación a lo visto en el teórico, defina: a)Tolerancia 12 Etilenglicol b) Dependencia Física: 2- ¿Cuales son las diferentes patologías producidas por el alcohol? (indique por lo menos dos de cada una: a) nutricionales: b) gastrointestinales: c) musculares: d) metabólicas: e) pulmonares: f) hematológicas: g) SNC: 3- Defina el Sme. de abstinencia, por que se produce y cual es el primer síntoma en aparecer. 4- Defina el Delirium tremens, cuales son sus síntomas y cuales sus posibles complicaciones. Dependencia vs. SME de Abstinencia. ¿Mismo síndrome, mismo tratamiento? Lea atentamente los artículos anexados a la guía de trabajos prácticos para poder contestar las siguientes preguntas. Benzodiazepine treatment for alcohol-dependent patients. 5- ¿Sobre cuales receptores actúa el etanol en el SNC? 6- ¿Por que las BZD serian el fármaco indicado para el SME de abstinencia? 7- Realice un cuadro comparativo entre los efectos del etanol y las benzodiacepinas sobre el SNC. ETANOL BZD GABA ____________________________________________________________________________ NA ____________________________________________________________________________ Eje HPA ____________________________________________________________________________ 13 8- ¿Cuáles serían las indicaciones de las BZD en el SME de Abstinencia? ¿Cual es la BZD de elección y cual debería ser su vía de administración? Drug Therapy for Alcohol Dependence 9- ¿Sobre cuáles receptores actuaría el alcohol llevando a la dependencia? 10- Nombre por lo menos 3 criterios que diagnostiquen Dependencia. 11- ¿Cuáles son los dos grupos de drogas aceptadas por la FDA? 12- Justifique por qué antiguamente se utilizaba el Disulfiram. ¿Cuáles son las precauciones que se debian tener con respecto a su uso? 13- Justifique el uso de Naltrexona. ¿Cuál es la dosis? ¿Qué precauciones se deben tener con respecto a su uso? SAF: 14- ¿Qué es el SAF? 15 - Describa las alteraciones físicas y mentales que produce. Artículo: Drug Therapy for Alcohol Dependence Robert M. Swift, M.D., Ph.D. Alcohol dependence is a chronic disorder that results from a variety of genetic, psychosocial, and environmental factors.1 As defined by the American Psychiatric Association in the Diagnostic and Statistical Manual of Mental Disorders, it is characterized by increased tolerance of the effects of alcohol, impaired control over drinking, and continued drinking despite adverse consequences 2 Alcohol dependence affects nearly 10 percent of the population and results in social problems, considerable morbidity and mortality, and high health care costs.3,4 14 Alcohol dependence is treated by medical, psychological, and social interventions that reduce or eliminate the desire to drink and the harmful effects of alcohol. Treatment usually consists of two phases: detoxification and rehabilitation. Detoxification ameliorates the symptoms and signs of withdrawal; rehabilitation helps the patient avoid future problems with alcohol. Most rehabilitative treatments are psychosocial, consisting of individual and group therapy, residential treatment in alcohol-free settings, and self-help groups such as Alcoholics Anonymous. Almost all programs advocate complete abstinence from alcohol. Although psychosocial treatments are effective in reducing alcohol consumption and in maintaining abstinence in many patients, 40 to 70 percent of patients resume drinking within a year after treatment.5 There is increasing interest in drug therapy for alcohol dependence.6,7,8 The rationale for such therapy is based on several premises. First, advances in neurobiology have identified neurotransmitter systems that initiate and maintain the drinking of alcohol; pharmacologic modification of these neurotransmitters or their receptors may modify dependence. Second, new drugs that reduce alcohol consumption in animals may also reduce consumption in humans. Third, the development of drugs for the treatment of addictive disorders, such as nicotine and opioid dependence, suggests that it may be possible to develop drugs for the treatment of alcohol dependence. In the United States, the Food and Drug Administration (FDA) has approved two drugs, disulfiram (Antabuse, Wyeth–Ayerst, Philadelphia) and naltrexone (ReVia, Dupont Merck, Wilmington, Del.), for the treatment of alcohol dependence. Acamprosate (Campral, Lipha, Lyons, France, and Merck, Darmstadt, Germany), approved in several European countries, is being tested in the United States. Tiapride (marketed by various manufacturers) is also approved in several European countries. Other drugs marketed as mood stabilizers, sedatives, anxiolytics, and antidepressants have been used to treat alcohol dependence. This review discusses the putative mechanisms of action of these drugs and their efficacy. The Neurobehavioral Aspects of Alcohol Dependence Alcohol is a drug with complex behavioral effects that can be pleasurable or unpleasant, stimulating or sedating. The predominant effects depend on the dose, the length of time after ingestion, whether ingestion is chronic or intermittent, the drinker's expectations, the setting in which alcohol is consumed, the drinker's personality, and his or her genetic predisposition to alcohol dependence. Alcohol affects several brain neurotransmitters, including dopamine, -aminobutyric acid, glutamate, serotonin, adenosine, norepinephrine, and opioid peptides, and their receptors. 7,8 Several neurobehavioral effects of alcohol have been related to the development of alcohol dependence (Table 2). These effects and their associated neurotransmitters are potential targets for drug therapy to treat dependence. The pleasurable and stimulant effects of alcohol are mediated by a dopaminergic pathway projecting from the ventral tegmental area to the nucleus accumbens.9,10 Repeated excessive alcohol ingestion sensitizes this pathway and leads to the development of dependence.11,12 Drugs that target this dopamine system may reduce the reinforcing effects of alcohol and thereby reduce alcohol consumption. Similarly, drugs that increase the aversive effects of alcohol may reduce consumption. People who are more sensitive to the sedative and aversive effects of alcohol appear less likely to drink heavily and to develop alcohol dependence.13 Drugs that reduce the acute and chronic symptoms of alcohol withdrawal may treat dependence by reducing the need for alcoholdependent patients to ingest alcohol to avoid this state.14 Long-term exposure to alcohol causes adaptive changes in several neurotransmitter systems, including down-regulation of inhibitory neuronal -aminobutyric acid receptors,15 up-regulation of excitatory glutamate receptors,16 and increased central norepinephrine activity.17 Discontinuation of alcohol ingestion leaves this excitatory state unopposed, resulting in the nervous system hyperactivity and dysfunction that characterize alcohol withdrawal. People who drink alcohol over long periods and excessively also have craving, defined as the conscious desire or urge to drink alcohol. Intense craving leads to a preoccupation with alcohol and increases the probability of drinking.18 Craving can occur in several circumstances: before alcohol ingestion, during alcohol ingestion, during acute withdrawal, and during exposure to the sight or smell of alcohol long after drinking has stopped. Craving has been linked to dopaminergic, serotonergic, and opioid systems that mediate positive reinforcement and to -aminobutyric acid, 15 glutamatergic, and noradrenergic systems that mediate withdrawal.14 Reductions in craving are associated with longer abstinence.19,20 Finally, it is proposed that some persons with psychiatric disorders become dependent on alcohol as a result of self-medication with alcohol to reduce psychiatric symptoms and distress.21 Alcohol dependence is common among patients with schizophrenia, panic disorder, and depression.22 Drugs that effectively treat the underlying psychiatric disorder may reduce the impetus for alcohol ingestion. Drug Treatment for Alcohol Dependence Most studies of drug treatment for alcoholism compare differences in drinking outcomes between treatment with the drug and with placebo in recently abstinent patients who are also receiving psychosocial therapy. Typical outcomes include increases in abstinence, expressed as the proportion of patients remaining abstinent or the length of time to the loss of abstinence (relapse), and reductions in the quantity or frequency of drinking, expressed as the number of drinking days or the number of drinks per drinking day. Although abstinence is the more stringent outcome and is preferred, reductions in consumption can nevertheless reduce alcohol-related morbidity. The drugs discussed below have been evaluated in double-blind, placebo-controlled clinical trials Aversive Drugs Alcohol metabolism is a two-stage process. Ethanol is converted to acetaldehyde by alcohol dehydrogenase, and acetaldehyde is then converted to acetate by aldehyde dehydrogenase (Figure 1). For most people ingesting alcohol, acetaldehyde is metabolized rapidly and efficiently, so that it does not accumulate. When it does accumulate, it causes tachycardia, flushing, diaphoresis, dyspnea, nausea, and vomiting. Inhibition of aldehyde dehydrogenase by disulfiram, an irreversible inhibitor, or calcium carbimide, a shorter-acting, reversible inhibitor, causes the accumulation of sufficient acetaldehyde to cause symptoms. The possibility of having these unpleasant symptoms provides a deterrent to alcohol ingestion. Although disulfiram has been reported to be an effective treatment for alcohol dependence in case reports and open-label studies, placebo-controlled clinical trials have been inconclusive.23,24 In the most rigorous and best-controlled double-blind treatment study, in which 605 alcohol-dependent men were treated with 250 mg of disulfiram daily, 1 mg of disulfiram daily (a pharmacologically ineffective dose), or no drug for a year, there were no differences in rates of abstinence or the length of time to a first drink among the three groups.25 Only men receiving 250 mg of disulfiram who ingested alcohol and became ill subsequently drank less alcohol than those in the other two groups. The authors concluded that disulfiram neither improves the rate of continuous abstinence nor delays the resumption of drinking, but that it may reduce drinking after relapse. In spite of its lack of efficacy as compared with placebo, some physicians and patients believe that disulfiram is an effective psychological deterrent to drinking. The usual dose of disulfiram is 250 mg per day, although doses of 125 mg to 1000 mg are sometimes given, depending on side effects and response. Some patients take disulfiram only when they are at high risk for relapse; others take it continuously. Administration of disulfiram under direct observation reportedly increases its effectiveness.26 Patients taking disulfiram must be aware of the danger of consuming alcohol in beverages, foods, over-the-counter medications, or mouthwashes. Disulfiram inhibits the metabolism of several medications, notably anticoagulant drugs, phenytoin, and isoniazid, thereby exaggerating their actions and toxic effects. It should be given cautiously to patients with liver disease and is contraindicated in pregnant women and patients with ischemic heart disease. Disulfiram can cause hepatitis, and liver-function tests should therefore be performed regularly during treatment. Opioid Antagonists The observation that µ-opioid (morphine-like) agonists increased alcohol consumption and that µopioid antagonists reduced alcohol consumption in animals28,29 led to clinical trials of naltrexone in patients with alcohol dependence. It has been proposed that naltrexone reduces drinking and increases abstinence by reducing the positively reinforcing, pleasurable effects of alcohol and by reducing the craving for alcohol. Naltrexone and other µ-opioid antagonists block the alcoholinduced release of dopamine in the nucleus accumbens.30,31 Social drinkers report less positive and more sedative and unpleasant effects of alcohol when taking naltrexone.32 Patients with alcoholism who drink during treatment with naltrexone report experiencing less alcohol "high" and are less 16 likely to progress to heavy drinking.33 Naltrexone also reduces the craving for alcohol in both alcoholic patients34 and social drinkers.35 Several double-blind, placebo-controlled trials have found that naltrexone is efficacious when combined with psychosocial treatments for alcohol dependence. In a 12-week, placebo-controlled, randomized clinical trial of 50 mg of naltrexone daily in 70 alcohol-dependent men receiving outpatient psychotherapy, the patients receiving naltrexone had significantly fewer drinking days than those given placebo (4 percent vs. 14 percent of study days), and fewer later resumed heavy drinking, defined as consuming five or more drinks in a day (23 percent vs. 54 percent of the men).36 In a double-blind study of 97 alcohol-dependent men and women given 50 mg of naltrexone or placebo per day and assigned either to therapy targeted to increasing individual coping skills and relapse prevention or to supportive therapy for 12 weeks, alcohol consumption was lower and the abstinence rate was higher among the naltrexone-treated patients.37 Fewer than half the patients in the naltrexone group resumed heavy drinking, as compared with more than 80 percent of those in the placebo group. Naltrexone was most effective for patients who reported strong cravings at study entry.38 An interaction between medication and psychotherapy was found, in that naltrexone increased abstinence among patients assigned to receive supportive psychotherapy but not among those assigned to psychotherapy designed primarily to increase coping skills. However, for patients taking naltrexone who drank, those who received coping-skills training were less likely to drink heavily than those who received supportive therapy. Other studies of naltrexone found no evidence of efficacy. In a randomized trial, 108 patients with alcohol dependence and cocaine and opiate abuse received both psychosocial therapy and either placebo or 50 or 100 mg of naltrexone daily; naltrexone did not alter drinking.39 Similarly, in a randomized, double-blind trial in the United Kingdom, there were no differences in drinking-related outcomes among 175 patients with alcoholism who were assigned to minimal psychosocial treatment and received either 50 mg of naltrexone per day or placebo for 12 weeks.40 In a randomized, double-blind, placebo-controlled trial of 50 mg of naltrexone per day or placebo in 64 patients with combined alcohol and cocaine dependence, naltrexone was no more beneficial than placebo.41 There are several possible reasons for the positive effects of naltrexone on drinking outcomes found in some studies and the lack of effect in others. One of the negative studies of naltrexone41 was probably too small to detect differences between the drug and placebo groups for a drug with a moderate effect. In two of the negative studies,39,41 the patients abused multiple substances, which may have limited the efficacy of naltrexone. Another important variable is compliance with medication. In the negative study in the United Kingdom, naltrexone significantly decreased the total number of drinks consumed and the number of drinks per day, as compared with placebo, but only among patients who took at least 80 percent of the prescribed medication. 40 The importance of compliance is supported by a U.S. study comparing naltrexone with placebo in 97 patients with alcoholism who also received weekly counseling. Those who were more compliant with naltrexone therapy were significantly less likely to report episodes of heavy drinking than those assigned to placebo (14 percent vs. 52 percent) and had fewer drinking days (2.8 percent vs. 11 percent), whereas the results among the noncompliant patients did not differ from those in the placebo group.42 For the first 90 days of abstinence, when the risk of relapse is greatest, the recommended dose of naltrexone is 50 mg once daily, but doses of 25 to 100 mg daily are sometimes used. The most common side effects are nausea (10 percent), headache (7 percent), anxiety (2 percent), and sedation (2 percent).43 The ingestion of naltrexone results in insensitivity to opioid drugs for 72 hours; if an opiate analgesic drug is required in an emergency, administration of a higher dose of opiates can overcome this insensitivity, but respiratory monitoring is mandatory. Although doses of 300 mg of naltrexone daily have been associated with hepatotoxic effects, such effects are rare at daily doses of 50 mg.44 Indeed, serum aminotransferase concentrations are often lower in patients given naltrexone than those given placebo, presumably because of their decreased alcohol ingestion.38 Nevertheless, patients with liver disease should be given naltrexone cautiously, and their liver function should be monitored periodically throughout treatment.45 Nalmefene, a µ- and -opioid antagonist, which is approved by the FDA for the reversal of opioid intoxication and overdose, is chemically similar to naltrexone but less hepatotoxic.46 In a 12-week clinical study of 21 patients with alcohol dependence who were given daily doses of 10 mg of 17 nalmefene, 40 mg of nalmefene, or placebo, the higher-dose nalmefene group was more abstinent than the other two groups.47 Dopaminergic Drugs Given the theoretical importance of dopamine in the neurobiology of alcohol dependence, there is interest in dopaminergic drugs as treatments for alcohol dependence. In animals, dopamine agonists and antagonists both decrease the stimulant and positively reinforcing properties of alcohol and decrease alcohol consumption. Dopamine antagonists can block the reinforcing effects of alcohol64; agonists may alleviate a dopamine-deficiency state.65 Tiapride, a dopamine D2-antagonist drug marketed in Europe as an atypical neuroleptic and anxiolytic drug, reduces the symptoms of alcohol withdrawal and is approved for the treatment of acute and chronic alcoholism.66 Its efficacy in patients with alcohol dependence has been evaluated in three clinical trials. In the largest, 100 recently abstinent alcohol-dependent patients were randomly assigned to receive 300 mg of tiapride per day or placebo for three months. Although only 54 percent of subjects complied with medication for at least one month, those who did and who received tiapride were more likely to remain abstinent and had lower rates of use of health care services.67 The dopamine agonist bromocriptine, used in the treatment of Parkinson's disease, was initially reported to reduce drinking in patients with alcoholism.68 However, a long-acting injectable bromocriptine preparation was no more effective than placebo in preventing relapses of drinking in 366 alcohol-dependent patients.69 Conclusions In patients with alcohol dependence, drug therapy can improve the outcomes of treatment and thereby reduce morbidity and mortality and improve the quality of life. Questions remain about the optimal drug dosage, the duration of treatment, concomitant psychosocial therapy, the cost effectiveness of drug therapy, and the types of patients who will benefit most from a specific drug. 18