* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Biochemistry notes File
Biomolecular engineering wikipedia , lookup
Synthetic biology wikipedia , lookup
Natural environment wikipedia , lookup
Developmental biology wikipedia , lookup
Photosynthesis wikipedia , lookup
Abiogenesis wikipedia , lookup
Evolutionary history of life wikipedia , lookup
History of biology wikipedia , lookup
Living things in culture wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
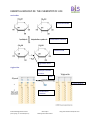
ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE 1. What are the three most commonly occurring elements? carbon (C), Hydrogen (H) and Oxygen (O) [the fourth is nitrogen (N)] 2. State one function of each of these elements: a. Iron function: Used in all organisms in enzymes in aerobic respiration b. Sulphur function: A part of two amino acids, essential in most proteins. c. Calcium function: Important messenger in cells including transcription of DNA. Also in nerve impulses, muscle contraction and bone formation in animals. d. Phosphorous function: Part of the phosphate group in ATP and DNA molecules. e. Sodium function: important in osmotic regulation of cells. Also important for nerve impulses and muscle contractions in animals. 3. Define organic molecule. Organic molecule is a molecule containing carbon and is found in living organisms. 4. What are three carbon-containing groups or molecules that are not organic? Hydrogen carbonates, carbonates and oxides of carbons are considered to be inorganic. 5. In the space below, draw three water molecules attracted to one another by hydrogen bonding. Include labels to show the polarity of the molecules. Hydrogen bond between the slightly negative oxygen atom in one water molecule and slightly positive Hydrogen atom in another water molecule Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School http://sciencevideos.wordpress.com ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE 6. Water has many properties which are essential for life. Complete the table below. Explanation Significance to living things Water is slow to gain and lose heat. Organisms can keep the temperature even if the environment increase or decrease the temperature temporarily. This is especially true for organisms living in or close to water habitat. The denser water at 4oC allows animals to survive at the bottom of lakes during the winter. The ice floats and insulates the water below. Mammals sweat, and plants in deserts increase their transpiration, for thermoregulation and preventing overheating. Habitat for Insects like the Pond Skaters (Skräddare). They can walk on the surface film and feed on small organisms trapped by surface tension of film. Female mosquitoes land on water surface and lay egg raft which floats. Watery habitat dissolves ions that can be absorbed by organisms. Soluble excretory products can be removed, for example urea). Soluble substances can be transported within the cytoplasm and in circulatory systems. Thermal Properties Water freezes at 0oC and is most dense at 4oC. Ice is less dense than water. Water requires a lot of energy to evaporate (to break the bonds between the molecules). Cohesion Solvent Properties Hydrogen bonds link water molecules together. This creates a “skin” on the surface of water, surface tension. Many substances can form hydrogen bonds with water and therefore dissolve. Examples are sugars. Ionic compounds may form ions in water and dissolve. Example is sodium chloride (NaCl) which forms sodium ions Na+ and chloride ions Cl-. (include uses as a coolant, medium for metabolic reactions, transport medium) 7. In the space below, draw the structure of a general amino acid. Include (and label) the amino, carboxyl and ‘R’ groups. amino group carboxyl group R group Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School http://sciencevideos.wordpress.com ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE 8. In the space below, draw the generalized structures of fatty acids and glycerol. 9. Draw the structures of glucose and ribose. ribose glucose 10. Complete the table below: -saccharides examples Plant or animal? Both Glucose Plant Mono- Di- Poly- Function/ uses Broken down in cell respiration to release energy Part of sucrose that transport the products from photosynthesis to storing organs of plants. Galactose Animal Fructose Plant Lactose Animal Sucrose plant The transport form of the products from photosynthesis to storage organs or where it will be used. Maltose animal Dimer of glucose, broken down from starch. Starch plant Glycogen animal Cellulose plant Insoluble storage of glucose Insoluble storage of glucose energy storage in liver an muscles Insoluble compound - Main component of cell wall Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School ‘brain sugar’ – less sweet/ less soluble Fruit sugar is used to make fruits sweet-tasting, attracting animals to disperse seeds n the fruit Milk sugar that provides energy to young mammals http://sciencevideos.wordpress.com ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE 11. Using labeled diagrams, describe how a monosaccharide (glucose) is converted to a disaccharide (maltose). What is the name of this process? Condensation reaction or dehydration reaction glucose glucose 12. What is the name of the process through which polypeptides, polysaccharides or triglycerides are catabolised (broken down)? Hydrolysis reactions 13. On a separate sheet of paper, outline condensation and hydrolysis in proteins, saccharides and triglycerides. Label the reactions and bonds formed/ broken clearly. 2 amino acids Proteins Hydrolysis reaction reaction Condensation reaction H2O H2O 1 Dipeptide Peptide linkage Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School http://sciencevideos.wordpress.com ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE saccharides 2 monosaccharides condensation reaction 1 disaccharide glycosidic bond triglycerides Hydrolysis reactions reactions 3 H2O 3 Ester bondings Condensation reactions Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School http://sciencevideos.wordpress.com ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE Link Extra Figure Condensation reactions Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School http://sciencevideos.wordpress.com ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE 14. Triglycerides can have saturated or unsaturated fatty acids attached. Differentiate between saturated and unsaturated fats in terms of: a. Bonds in the fatty acid chain Saturated triglycerides only have single bonds in their fatty acids. Unsaturated triglycerides have double bonds in their fatty acids. b. Melting point/ state at room temperature Saturated triglycrides are solid at room temperature. Unsaturated triglycerides are liquid at room temperature. c. Origin (plants or animals) Saturated triglycerides are more common in animals, especially warm blooded (homoithermic) animals. Unsaturated triglycerides are more common in plants and in cold blooded (piokilothermic) animals. 15. State three functions of lipids: 1. Energy storage – fat in animals and oils in plants 2. Heat insulation – a layer of fat under the skin reduces heat loss 3. Boyancy – lipids are less dense than water so help animals to float Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School http://sciencevideos.wordpress.com ESSENTIAL BIOLOGY 03: THE CHEMISTRY OF LIFE 16. Compare lipids and carbohydrates in terms of energy storage: carbohydrates lipids Starch in plants and glycogen in animals Triglycerides or fat in animals and oils in plants Short time storage Long time storage Stored as…? Long/short term storage? Ease of digestion/ release of energy? Easily broken down to release More complex and so slower to energy quickly. brake down. Energy per gram? 17 kJ per gram 38 kJ per gram Solubility in water? (and consequence) Soluble in warm water and insoluble in cold water Insoluble in water Use of oxygen in metabolism? (and consequence) May be used for anaerobic Only for aerobic respiration respiration temporarily in muscle cells. In this case lactic acid will be produced. Essential Biology Revision Series (Chem of Life, inc. AHL and Option C) Steve Taylor Bandung International School http://sciencevideos.wordpress.com