* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download phosphate buffer system
Developmental biology wikipedia , lookup
Cell theory wikipedia , lookup
List of types of proteins wikipedia , lookup
Animal nutrition wikipedia , lookup
Biochemistry wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Homeostasis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Membrane potential wikipedia , lookup
Magnesium in biology wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
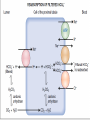
HUMAN RENAL SYSTEM PHYSIOLOGY Lecture 9,10 BY: LECT. DR. ZAINAB AL -AMILY objectives • Describe Acid Base balance Acid-Base Balance • Metabolic activities of the body require precise regulation of acid-base balance, which is reflected by the pH of ECF. • pH being regulated within a narrow physiologic range affect: – membrane excitability – enzyme systems – chemical reactions. • Many conditions, pathologic or otherwise, can alter body pH. • Normally, the concentration of body acids and bases is regulated so that the pH of ECF is maintained within a very narrow range of 7.35 to 7.45. • This balance is maintained through mechanisms that: generate Buffer acids and bases eliminate. • An acid is a molecule that can release a hydrogen ion (H+) • A base is a molecule that can accept or combine with an H+ ion. • Most of the body’s acids and bases are weak acids and bases. • Most important are carbonic acid (H2CO3), which is a weak acid derived from carbon dioxide (CO2), and bicarbonate (HCO3-), which is a weak base. • The concentration of H+ ions in body fluids is low compared with other ions. i.e., the Na+ ion present at a concentration approximately 1 million times that of the H+ ion. • Because of its low concentration in body fluids, the H+ ion concentration is commonly expressed in terms of pH. • Specifically, pH represents the negative logarithm (p) of the H+ ion concentration in mEq/L. • A pH value of 7.0 implies a H+ ion concentration of 10−7 (0.0000001 mEq/L). • pH is inversely related to the H+ ion concentration • Low pH indicates a high concentration of H+ ions and a high pH, a low concentration. • Metabolic Acid and HCO3- Production • Acids are continuously generated as by-products of metabolic processes. • acids fall into two groups: a. volatile acid (H2CO3) • H2CO3 is in equilibrium with the volatile CO2, which leaves the body by way of the lungs. • H2CO3 concentration is determined by the lungs and their respiratory capacity. b. nonvolatile or fixed acids. • e.g., sulfuric, hydrochloric, phosphoric are not eliminated by the lungs. • Instead, they are buffered by body proteins or extracellular buffers, such as HCO3- , and then excreted by the kidney. • CO2 and HCO3- Production • Body metabolism results in the production of approximately 15,000 mmol of CO2 each day. • CO2 is transported in the circulation in three forms: (1) attached to hemoglobin (2) as dissolved CO2 (i.e., PCO2) (3) HCO3- • Dissolved CO2 and HCO3- constitute: – 77% of the CO2 that is transported in ECF – remaining CO2 attached to Hb. • Small % of CO2 combines with water in bloodstream H2CO3 catalyzed by carbonic anhydrase (present in large quantities in RBCs, renal tubular cells, and other tissues in the body). • It is impossible to measure H2CO3 dissolved CO2 measurements are commonly substituted when calculating pH. • Calculation of PH • pH is calculated with the Henderson-Hasselbalch equation using the dissociation constant for the bicarbonate buffer system (6.1) and the HCO3- to H2CO3 ratio. • pH = 6.1 + log HCO3-/H2CO3 (CO2) • The log of 20 is 1.3. Thus, when the ratio is 20 to 1, the pH is within the normal range at 7.4. • The generation of metabolic acids and the availability of bicarbonate to buffer these acids control the HCO3- part of the equation. The H2CO3 part of the equation is regulated by the lungs and their ability to eliminate CO2. The kidney functions in the generation and reabsorption of HCO3- and contributes to control of the metabolic part of the equation. Because the ratio is used, a change in HCO3- will have little or no effect on pH, as long as there are accompanying changes in H2CO3. • Acidification of Urine and Bicarbonate Excretion: • Cells of the proximal and distal tubules as well as those of the collecting ducts, like cells of the gastric glands, secrete H+ ions: • The reaction in the proximal tubule is an example of a secondary active transport by the Na+ - H+ antiport (exchanger). • As in the figure, extrusion of Na+ from the cell by (Na+–K+ ATPase) pump lowers the intracellular (IC) Na+, causing Na+ to enter the cell from the tubular lumen, with coupled extrusion of H+ ions. • The H+ ion comes from the IC-dissociation of H2CO3 into HCO3¯ and H+, reaction that is catalyzed by carbonic anhydrase CA enzyme (5000 times). • The bicarbonate ion that is formed diffuses into ISF, along with Na+ ions (through Na+–HCO3¯ cotransporter) for each H+ ion that is secreted. • In the late distal tubule and the collecting ducts, H+ ion secretion is relatively independent of Na+ ion in the tubular lumen. • aldosterone hormone act on the I- cells that are responsible for secreting acid in this part of the tubule, and will activate an ATP-driven proton pump to increase the distal H+ ion secretion. • The I- cells contain an ion exchanger protein that is called “band 3” in their basolateral cell membranes, which functions as a (Cl¯ – HCO3¯ exchanger) for the transport of HCO3¯ into ISF. • Finally, some of the H+ ions are also secreted by the (H+ – K+ ATPase) pump. • When the number of free H+ ions secreted into the tubular fluid threatens to cause the urine to become too acidic, the H+ ions must be carried in some other form. • This is accomplished by combining the H+ ions with intratubular buffers before being excreted in the urine. • There are two important intratubular buffer systems: 1. The phosphate buffer system 2. The ammonia buffer system. • phosphate buffer system • It uses HPO42- and H2PO4- that are present in the tubular filtrate to buffer H+. • The combination of H+ with HPO42to form H2PO4- allows the kidneys to increase their secretion of H+ ions. • Because they are poorly reabsorbed, the phosphates become more concentrated as they move through the tubules. • Ammonia buffer system • Renal tubular cells are able to use the amino acid glutamine to synthesize ammonia (NH3) and secrete it into the tubular fluid. • H+ ions combine with the NH3 to form an ammonium ion (NH4+). • NH4+ ions combine with Cl- ions (Cl-), which are present in the tubular fluid, to form ammonium Cl(NH4Cl), which is then excreted in the urine. • Regulation of pH • The pH of body fluids is regulated by three major mechanisms: 1. ICF and ECF buffering systems 2. the lungs, which control the elimination of CO2 3. the kidneys, which eliminate H+ and regulate the elimination of HCO3- • IC and EC Buffer Systems • The moment by-moment regulation of pH depends on the ICF and ECF buffer systems. • A buffer system consists of a weak acid and the base salt of that acid or of a weak base and its acid salt. • In the process of preventing large changes in pH, the system swap a strong acid for a weak acid or a strong base for a weak base. • Major buffer systems protect the pH of body fluids are: 1. Proteins 2. HCO3- buffer system 3. Transcellular H+/K+ exchange system. • These buffer systems are immediately available 4.Bone also represents an important site for the buffering of acids and bases (for long term ). 1.Proteins • Are the largest buffer system in the body. • Albumin and plasma globulins are the major protein buffers in the vascular compartment. • are amphoteric, meaning that they can function as either acids or bases. • They contain many ionizable groups that can release or bind H+. • are largely located within cells, and H+ ions and CO2 diffuse across cell membranes for buffering by intracellular proteins 2.bicarbonate buffer system • uses H2CO3 as its weak acid and HCO3- as its weak base. • It substitutes the weak H2CO3 for a strong acid such as HCl or the weak HCO3- base for a strong base such as sodium hydroxide. • HCO3-/H2CO3 buffer system is an efficient system because the buffer components can be readily added or removed from the body. • Metabolism provides an sufficient supply of CO2 as body requirement • The kidney can form new HCO3- when excess acid is added, and it can excrete HCO3- when excess base is added. 3.Transcellular H+/K+ exchange system • Both ions are positively charged, and both ions move freely between the ICF and ECF compartments. • When excess H+ is present in the ECF, it moves into the body cells in exchange for K+. • When excess K+ is present in the ECF, it moves into the cell in exchange for H+. • On the average, serum K+ rises by approximately 0.6 mEq/L for every 0.1 unit fall in pH. • Thus, alterations in K+ levels affect acid-base balance, and changes in acid-base balance influence K+ levels. • In acidosis, increased levels of serum K+ cause the resting membrane potential to become less negative and • in alkalosis, decreased levels cause the resting membrane potential to become more negative. • Changes in neural excitability are further influenced by alterations in ionized Ca2+. • In acidosis: – ionized portion of the extracellular Ca2+ is increased making neurons less excitable • In alkalosis: – amount of ionized Ca2+ is reduced making neurons more excitable. 1. Secretion of K+ by the distal tubule will be decreased by (A) metabolic acidosis (B) a high-K+ diet (C) hyperaldosteronism (D) spironolactone administration (E) thiazide diuretic administration • Respiratory Control Mechanisms • provides for the elimination of CO2 into the air and plays a major role in acid-base regulation. • The respiratory control of pH is rapid, occurring within minutes, and is maximal within 12 to 24 hours. • it does not return the pH to normal. • Renal Control Mechanisms • The kidneys regulate acid-base balance by excreting acidic or alkaline urine. • The renal mechanisms CANNOT adjust the pH within minutes, as respiratory mechanisms can, but they continue to function for days until the pH has returned to normal or near-normal range. • The kidneys filter HCO3- in the glomerulus and then reabsorb it in the tubules as a means of maintaining ECF levels. • Almost 99.9% of the filtered HCO3 is reabsorbed • The reabsorption rate can be calculated by comparing the filtered load of HCO3 with the excretion rate of HCO3 • If the glomerular filtration rate (GFR) is 180 L/day and the plasma HCO3 concentration is 24 mEq/L, then the filtered load is 4320 mEq/day (180 L/day × 24 mEq/L). • The measured excretion rate of HCO3 is merely 2 mEq/day. Therefore, the reabsorption rate of HCO3 is 4318 mEq/day, which is 99.9% of the filtered load. • Most filtered HCO3 reabsorption occurs in the proximal tubule and only small quantities are reabsorbed in the loop of Henle, distal tubule, and collecting duct. • HCO3- do not readily cross the membranes of renal tubular cells; HCO3- that has been filtered in the glomerulus cannot be directly reabsorbed. • Instead, HCO3- ions first combine with H+ ions that have been secreted into the tubular fluid to form H2CO3, which is converted to CO2 and water. • The resulting CO2 can readily cross the tubular membrane and enter the tubular cell, where it combines with water, under the influence of carbonic anhydrase, to generate a new H2CO3 molecule. • The newly formed H2CO3, in turn, dissociates to form a HCO3- and a H+ ion. • The HCO3- that is formed moves out of the tubular cell into the bloodstream and the H+ is secreted into the tubular fluid. • Aldosterone also influences H+ ion elimination by the kidney. • It acts in the collecting duct to indirectly stimulate H+ ion secretion, while increasing Na+ ion reabsorption and K+ secretion. • Hyperaldosteronism tends to lead to a decrease in serum K+ levels and increased pH and alkalosis caused by increased H+ ion secretion. • Hypoaldosteronism leads to increased K+ levels, decreased H+ ion secretion, and acidosis. • One of the mechanisms that the kidneys use in regulating the pH of the ECF is the conservation or elimination of HCO3 -ions. • Cl- is the most abundant anion in the ECF and can substitute for HCO3- when an anion shift is needed. • i.e., serum HCO3- levels normally increase as (HCl is secreted into the stomach after a heavy meal, causing what is called the postprandial alkaline tide. • Later, as the Cl- ion is reabsorbed in the small intestine, the pH returns to normal.





































![Regulation of [H+] - Rowdy | Rowdy | MSU Denver](http://s1.studyres.com/store/data/008280740_1-c0ee3ef824bd0df09bfb225bc03a6632-150x150.png)





![ACID-BASE BALANCE Acid-base balance means regulation of [H + ]](http://s1.studyres.com/store/data/000604092_1-2059869358395bda26ef8b10d08c9fb9-150x150.png)




