laser1
... energized, or excited to specific energy levels. • More atoms or molecules are in a higher excited state. • The process of producing a population inversion is called pumping. • Examples: →by lamps of appropriate intensity →by electrical discharge ...
... energized, or excited to specific energy levels. • More atoms or molecules are in a higher excited state. • The process of producing a population inversion is called pumping. • Examples: →by lamps of appropriate intensity →by electrical discharge ...
Document
... • (-ve sign for DE indicates emission (+ve for absorption) • since energy (wavelength, frequency) of light can only be +ve it is best to consider such calculations as DE = Eupper - Elower C has been found from experiment. It is now called R, the Rydberg constant. R = 1312 kJ/mol or 3.29 x 1015 Hz ...
... • (-ve sign for DE indicates emission (+ve for absorption) • since energy (wavelength, frequency) of light can only be +ve it is best to consider such calculations as DE = Eupper - Elower C has been found from experiment. It is now called R, the Rydberg constant. R = 1312 kJ/mol or 3.29 x 1015 Hz ...
Simple harmonic motion= motion that repeats itself in an identical
... units (such as the Planck length and the Planck mass), all based on fundamental physical constants. In the photoelectric effect, electrons are emitted from matter (metals and non-metallic solids, liquids or gases) as a consequence of their absorption of energy from electromagnetic radiation of very ...
... units (such as the Planck length and the Planck mass), all based on fundamental physical constants. In the photoelectric effect, electrons are emitted from matter (metals and non-metallic solids, liquids or gases) as a consequence of their absorption of energy from electromagnetic radiation of very ...
“We choose to examine a phenomenon which is impossible
... The important results from last time: Quantum mechanical entities can exhibit either wave-like or particle-like properties, depending on what one measures. We saw this phenomenon for photons, and claimed that it is also true for matter (e.g., electrons). The wave and particle properties are related ...
... The important results from last time: Quantum mechanical entities can exhibit either wave-like or particle-like properties, depending on what one measures. We saw this phenomenon for photons, and claimed that it is also true for matter (e.g., electrons). The wave and particle properties are related ...
Einstein`s prediction
... •Energy fluctuations make the geometry of space-time fluctuate. At a small enough size scale, the assumption of a smooth, continuous space and time breaks down. ...
... •Energy fluctuations make the geometry of space-time fluctuate. At a small enough size scale, the assumption of a smooth, continuous space and time breaks down. ...
Set #4
... 1. How is the quantization of the energy in the hydrogen atom similar to the quantization of the systems discussed in the 1-D infinite quantum well? How is it different? Do the quantizations originate from similar causes? (Krane, Q8, pg. 201) 2. In both the Rutherford theory and the Bohr theory, we ...
... 1. How is the quantization of the energy in the hydrogen atom similar to the quantization of the systems discussed in the 1-D infinite quantum well? How is it different? Do the quantizations originate from similar causes? (Krane, Q8, pg. 201) 2. In both the Rutherford theory and the Bohr theory, we ...
Lecture 5. Radiation and energy. 1. The most important aspects of
... The energy of electrons in an atom can have only certain fixed values. The energies of the electrons are said to be quantized. Electrons restricted to the same allowed value of energy are said to occupy the same energy levels. All energy levels except the first one are divided into sublevels. There ...
... The energy of electrons in an atom can have only certain fixed values. The energies of the electrons are said to be quantized. Electrons restricted to the same allowed value of energy are said to occupy the same energy levels. All energy levels except the first one are divided into sublevels. There ...
Light/Electrons
... properties that are normally associated with waves. The wave properties are especially applicable to very small particles, such as electrons. Each particle’s wavelength is related to its mass, its velocity and Planck’s constant. Smaller the mass, and greater the velocity, the more wavelike the chara ...
... properties that are normally associated with waves. The wave properties are especially applicable to very small particles, such as electrons. Each particle’s wavelength is related to its mass, its velocity and Planck’s constant. Smaller the mass, and greater the velocity, the more wavelike the chara ...
Period 3 Solutions: Electromagnetic Waves – Radiant Energy II
... a) Connect a solar cell to the white amplifier/loudspeaker. What happens when an LED flashlight connected to a radio shines on the solar cell? What type of radiant energy transfers information? A modulated (changing) current from the radio transfers information by modulating the amplitude of the bea ...
... a) Connect a solar cell to the white amplifier/loudspeaker. What happens when an LED flashlight connected to a radio shines on the solar cell? What type of radiant energy transfers information? A modulated (changing) current from the radio transfers information by modulating the amplitude of the bea ...
Double-Slit Experiment
... Energy is quantized. It can occur only in discrete units called ______________. de Broglie: Corrected equation to account for relationship between mass and wavelength: m= de Broglie equation: ...
... Energy is quantized. It can occur only in discrete units called ______________. de Broglie: Corrected equation to account for relationship between mass and wavelength: m= de Broglie equation: ...
Atomic Structure 1. Historical perspective of the model of the atom a
... which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or rearranging atoms. b.) In 1910, Ernest Rutherford pa ...
... which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or rearranging atoms. b.) In 1910, Ernest Rutherford pa ...
unit 7 hw packet File
... electrons in one orbital will have opposite electron spin. Hund’s rule – electrons filling an orbital set (degenerate orbitals) will have the same spin and fill different orbitals until the orbital set is half full. Heisenberg uncertainty principle – can not know exact momentum (speed) and location ...
... electrons in one orbital will have opposite electron spin. Hund’s rule – electrons filling an orbital set (degenerate orbitals) will have the same spin and fill different orbitals until the orbital set is half full. Heisenberg uncertainty principle – can not know exact momentum (speed) and location ...
BasicQuantumMechanics18And20January2017
... • Inconsistency with classical light theory According to the classical wave theory, maximum kinetic energy of the photoelectron is only dependent on the incident intensity of the light, and independent on the light frequency; however, experimental results show that the kinetic energy of the photoele ...
... • Inconsistency with classical light theory According to the classical wave theory, maximum kinetic energy of the photoelectron is only dependent on the incident intensity of the light, and independent on the light frequency; however, experimental results show that the kinetic energy of the photoele ...
Document
... Example 28-1 (continued). The strong nuclear force has a range of about 1.5x10-15 m. In 1935 Hideki Yukawa predicted the existence of a particle named the pion (π) that somehow “carried” the strong nuclear force. Assume this particle can be created because the uncertainty principle allows non-conse ...
... Example 28-1 (continued). The strong nuclear force has a range of about 1.5x10-15 m. In 1935 Hideki Yukawa predicted the existence of a particle named the pion (π) that somehow “carried” the strong nuclear force. Assume this particle can be created because the uncertainty principle allows non-conse ...
Unit 8 Heat Study Guide A change of state is a ___ Process by
... 1. A change of state is a ___ a. Process by which two states of matter co-exist b. Chemical change c. Physical change that converts a substance from one physical form to another 2. Particles in a __________ move slower than particles in a __________. 3. Particles in a ___ vibrate in place. a. Solid ...
... 1. A change of state is a ___ a. Process by which two states of matter co-exist b. Chemical change c. Physical change that converts a substance from one physical form to another 2. Particles in a __________ move slower than particles in a __________. 3. Particles in a ___ vibrate in place. a. Solid ...
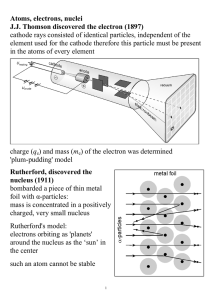
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... de Broglie (1923) described the discrete energy levels of electrons within an atom as results of a wave phenomenon momentum of an electron p = mev = h/p where λ is the wavelength of the matter wave corresponding to the electron, and h is the Planck constant. Davisson and Germer (1927) used electro ...
... de Broglie (1923) described the discrete energy levels of electrons within an atom as results of a wave phenomenon momentum of an electron p = mev = h/p where λ is the wavelength of the matter wave corresponding to the electron, and h is the Planck constant. Davisson and Germer (1927) used electro ...
Document
... Quantum mechanics: simple picture Consider simultaneous detection: 1. Both come from b 2. Both come from a 3. b->B and a->A (red) 4. b->A and a->B (green) ...
... Quantum mechanics: simple picture Consider simultaneous detection: 1. Both come from b 2. Both come from a 3. b->B and a->A (red) 4. b->A and a->B (green) ...
Ch # 17 Advent of Modern Physics Special Theory Of Relativity
... 3. (In the region of short wavelength, in the region of long wavelength, both in the region of short and long wavelength, none of these) 4. Wein’s theory is complete disagreement with the experimental curve of black body radiation __________. 5. (In the region of short wavelength, in the region of l ...
... 3. (In the region of short wavelength, in the region of long wavelength, both in the region of short and long wavelength, none of these) 4. Wein’s theory is complete disagreement with the experimental curve of black body radiation __________. 5. (In the region of short wavelength, in the region of l ...
Quantum Theory and Atomic Structure
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
PHY 410 Final Examination, Spring 2008 April 30, 2008 (5:45-7:45 p.m.)
... a constant energy depending on the crystal, and µ m is the magnetic moment associated with the state m . (15 points) a) Write down the partition function. b) What is the average magnetic moment M of the atom? Express your answer as a function of µ , B, ∆, T . c) When the magnetic field is very small ...
... a constant energy depending on the crystal, and µ m is the magnetic moment associated with the state m . (15 points) a) Write down the partition function. b) What is the average magnetic moment M of the atom? Express your answer as a function of µ , B, ∆, T . c) When the magnetic field is very small ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.