* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Einstein`s prediction
Quantum entanglement wikipedia , lookup
Renormalization wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Scalar field theory wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
History of quantum field theory wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Particle in a box wikipedia , lookup
Hydrogen atom wikipedia , lookup
Quantum teleportation wikipedia , lookup
Bell's theorem wikipedia , lookup
Canonical quantization wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Quantum key distribution wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Matter wave wikipedia , lookup
EPR paradox wikipedia , lookup
Atomic theory wikipedia , lookup
Hidden variable theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Wave–particle duality wikipedia , lookup
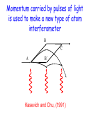
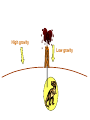
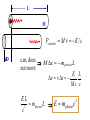
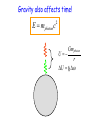
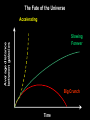
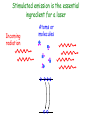
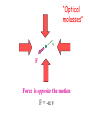
Einstein’s Legacy (and its relation to laser cooling and trapping) Chinese Academy of Sciences Beijing 13 December, 2006 “There are at least two reasons for giving a talk. Either the material is so new that no one has heard it, or else the material is so old that people have forgotten it.” R.H. Dicke 3rd International Conference on Quantum Electronics, 1964 Albert Einstein 1905 Einstein’s Legacy • Photons are quantized (photo-electric effect), carry momentum (special relativity) • Photons are affected by gravitational fields (General Theory of Relativity) • Stimulated emission and lasers • Brownian motion (the weighing of atoms and confinement in optical molasses) • Bose-Einstein condensation The spectrum of blackbody radiation The spectrum of light emitted from a blackbody is the same as the light emitted out of a hole in a metal cavity. According to classical physics, the energy in each mode of radiation can assume any value. Radiation intensity Classical calculation Measured spectrum Frequency In order to derive the frequency spectrum in a cavity filled with radiation that is consistent with experiment, Planck assumed that the energy at each frequency component is equal to = s , where s is an integer. “We consider, however – and this is the essential point of the whole calculation – [the energy] as made up of an entirely determined number of finite equal parts, and we make use of the natural constant h = 6.55 x 10-27 erg sec.” Max Planck If the energy is divided into an integer number of “quanta”, the possibilities are 0, 1, 2, 3 … S=3 S=3 S=2 The average number in thermal equilibrium s is found by summing the probabilities of finding the box with s photons. S=1 S=0 P( s) s s P ( s) s 0 The energy at frequency is E = s . exp( E / kBT ) exp( s / kBT ) • The energy spectrum is the sum of the thermal energy of light at each frequency. • Planck initially regarded his radical assumption at a mathematical “trick” to get the right answer …. Does light really come in integral units of energy? Photoelectric Effect Direct evidence for the quantization of light! Emax = h - Emax e- c h •Decreasing the intensity of light fewer electrons •Decreasing the frequency of light below a critical value c no electrons. Quantization of light quantization of photon momentum What is the momentum of each photon? Maxwell volume Density of of light electromagnetic Energy of the light pulse energy (ergs/cm3) pulse area of Radiation Momentum impulse light (t) (c) pressure = (dynes/cm of light pulse 2) pulse Momentum carried by pulses of light is used to make a new type of atom interferometer B C A B´ Kasevich and Chu, (1991) Measurement of gravity compared to Earth tide models Measured values of gravity compared to solid Measured acceleration, g (10-8 m/s2) earth tide model with and without ocean loading effects A. Peters, K.-Y. Chung and S. Chu, Nature (1999). 100 0 -100 Data Solid earth tide only With ocean loading -200 Residual acceleration (10-8 m/s2) 20 10 0 -10 -20 12 mn 12 noon 12 mn 12 noon 12 mn High gravity Low gravity • Photons are quantized (photo-electric effect), carry momentum (special relativity) • Photons are affected by gravitational fields (General Theory of Relativity) • Stimulated emission and lasers • Brownian motion (the weighing of atoms and confinement in optical molasses) • Bose-Einstein condensation Photons not only carry momentum, they transport mass How can this be true if the rest mass of a photon is zero? (rest mass of photon) E ( pc) 0 2 2 2 L M Pcylinder M v E / c x c.m. does M x m photon L not move E L x v t Mc c EL 2 m photon L E mphoton c 2 c a) b) Huge mass The equivalence of acceleration and gravity implies that light must bend in a gravitational field. Light travels in straight lines. Einstein used the path taken light as the definition of a straight line. Gravity also affects time! E mphoton c 2 U Gmphoton r U Newton’s Law of Gravity assumes space can be defined by a three dimensional geometry due to Euclid. In Euclidian geometry, the sum of the angles of a triangle add up to 180°. Without matter, space is described by Euclid’s geometry. Einstein’s General Theory of Relativity says mass distorts the geometry of space and time. Georg Fredrich Bernard Riemann In curved space, the angles can add up to be more or less than 180°, Or less than than 180°. Carl Frederick Gauss The vacuum has fields that fluctuate Evidence for the existence for quantum fluctuations: •Emission of light by atoms •Corrections to the energy levels of atoms allow predictions accurate to a part in a trillion. •The long range attraction of atoms to each other. The fundamental problem •The size of these quantum fluctuations increase inversely with respect to the region of space we look at. x p h •Energy fluctuations make the geometry of space-time fluctuate. At a small enough size scale, the assumption of a smooth, continuous space and time breaks down. This length scale is ~10-33 cm. One way out of this dilemma is to say that there is a smallest size of space and time that is allowed…the size of elementary “strings”. Quantum mechanics and the general theory of relativity The Fate of the Universe Accelerating Average distance between galaxies Slowing Forever Big Crunch Time 5th Solvay Conference, Brussels, 1927 The debates of Bohr and Einstein “Einstein came down to breakfast and expressed his misgivings about the new quantum theory. Every time [he] had invented some beautiful experiment from which one saw that [the theory] did not work … Pauli and Heisenberg, who were there did not pay much attention, “Ah well, it will be all right, it will be all right…” Bohr, on the other hand, reflected on it with care and in the evening … he cleared up the matter in detail.” Otto Stern recollection of the 1927 Solvay Meeting The Bohr-Einstein debate: Round II After the Solvay Conference, Einstein writes to Schrödinger a year later: “The soothing philosophy – or religion? – of Bohr and Heisenberg is so cleverly concocted that for the present it offers the believers a soft pillow from which they are not easily chased away… This religion does damn little for me.” Einstein’s strikes back with the EPR paradox. Leon Rosenfeld recalls: “This onslaught came down upon us as a bolt from the blue … as soon as Bohr heard my report of Einstein’s argument, everything else was abandoned: we had to clear up the misunderstanding at once … In great excitement, Bohr immediately started dictating to me the outline of such a reply. Very soon, however, he became hesitant; “No, this won’t do, we must try all over again…we must make it quite clear.” So it went on for a while, with growing wonder at the unexpected subtlety of the argument ...he would turn to me: “What can they mean? Do you understand it?” There would follow some inconclusive exegesis…” Bohm’s version of the Einstein-Podolsky-Rosen paradox A system with L = 0 decays into two particles, each carrying L = ½. When Alice measures +½, Bob must measure -½. The situation is analogous to the initial state consisting of a black and white marble. Bob is free to rotate his polarizer so that it measures spin along x or y, while Alice always measures along y. If Bob measures +x, then Alice must see –x. Since Alice measures along y, she has a 50-50 chance of getting -y. Now suppose Bob chooses to measure y and gets +y. Then Alice has to measure –y. The paradox: How can Bob affect Alice’s measurement? Quantum mechanics describes the state before any measurement as the combined (“entangled”) state |+xBob, -xAlice + |-xBob, +xAlice . The measurement “collapses” the state to either of the two states. Einstein: the system was already in one of the two states before Bob’s measurement. If Bob decided to rotate his polarizer at the last minute, he could not affect Alice’s measurement. There is an objective reality to Nature Causality demands that measurements separated by light years (space-like separation) can not affect each other. Quantum Mechanics provides probabilistic predictions of the outcome because it is incomplete. There may be “hidden variables”, and a complete knowledge of all the parameters of a system would make the theory deterministic. If these “hidden variables” were to remain permanently hidden, Einstein’s conjecture is not falsifiable. In 1964, John Bell derived an inequality using three angles of polarization that would differentiate Quantum Mechanics from any local hidden variable theory. Bell’s argument: •List all possible experimental outcomes of the two measurements. (Bob measures “this” and Alice measures “that”.) The states “this” and “that” existed before the measurement. •There are 8 choices allowed by physics. These choices can occur with any predetermined probability. •For appropriate choice of angles, Quantum Mechanics gives a different prediction, independent of the probabilities. Bell’s inequalities were tested in the 1970’2 and 1980’s. Clauser & Freedman, 1972 Aspect, Dalibard, and Roger, 1982 22.5° Local hidden variable prediction 67.5° Does the verification of Quantum Mechanics make the quantum measurement problem and EPR paradox go away? No! Quantum measurement is still a problem…and we still don’t understand it. • Photons are quantized (photo-electric effect), carry momentum (special relativity) • Photons are affected by gravitational fields (General Theory of Relativity) • Stimulated emission and lasers • Brownian motion (the weighing of atoms and confinement in optical molasses) • Bose-Einstein condensation Einstein used the Planck distribution to predict the existence of stimulated emission. E2, N2 A21 B12 u() B21 u() E1, N1 In order for the population of atoms to agree with the thermal distribution according to Boltzmann and Planck, there must be another process. Stimulated emission is the essential ingredient for a laser Incoming radiation Atoms or molecules In order to get more molecules in the excited state than in the ground state, Charles Townes (the co-inventor of the maser and the laser) came up with the following scheme: • Photons are quantized (photo-electric effect), carry momentum (special relativity) • Photons are affected by gravitational fields (General Theory of Relativity) • Stimulated emission and lasers • Brownian motion (the weighing of atoms and confinement in optical molasses) • Bose-Einstein condensation Brownian motion of a small particle in water. The constant jiggling of the particle was not due to the fact that it was alive! If the erratic motion was due to collisions with individual molecules of water, the early estimate of the mass of the water was to large by many orders of magnitude. • Einstein showed that Brownian motion was due to fluctuations in large number of molecules hitting from opposing sides. • There is also a viscous drag force acting on the particle. dv m dt v + F (t ) The solution to this differential equation gives x 2 2 k BT t 2D t This simple equation opens up the first quantitative way to measure the weight of a water molecule! A measurement of Boltzmann’s constant, the specific heat of a gas at constant pressure (CP) and volume (CV) determines Avogadro’s Number, NA, the number of molecules in 18 grams of water. CP Cv N A kB “Optical molasses” v F Force is opposite the motion F = - v 1 2 1 mv 2 2 1 2 1 mv 2 2 • Photons are quantized (photo-electric effect), carry momentum (special relativity) • Photons are affected by gravitational fields (General Theory of Relativity) • Stimulated emission and lasers • Brownian motion (the weighing of atoms and confinement in optical molasses) • Bose-Einstein condensation When atoms move slowly, they become big-fuzz balls d Einstein’s prediction: When d, the atoms will condense into a single gigantic wave The reason for Bose-Einstein condensation comes from fundamental ideas of statistical mechanics and quantum mechanics! Consider a small box of gas atoms that is released into a larger volume. The Second law of Thermodynamics • The entropy S of a closed system will remain constant or increase in time. The entropy of the atoms in the larger box will increase when no longer confined to the smaller box. S kB log number of accessible states • All atoms are equally likely to be found anywhere in the larger box. The most likely configurations have atoms more or less equally distributed. • A thermodynamic system tries to minimize its energy and maximize its entropy. •This trade-off is described in terms of minimizing the “Free Energy”, F of the system. F E TS As the atoms in our box are cooled to lower temperatures, the entropy S decreases. WHY? Quantum mechanics: = 2 h / p d As the atom fuzz balls get bigger, the number of accessible states decreases, decreasing the entropy S. In order that the Free Energy, F = E – TS not increase, a macroscopic number of atoms begin to drop into the lowest energy state. Atoms condensed into the lowest energy state. Einstein Faraday Newton Maxwell How can mere mortals hope to make contributions that compare to these Giants? “The knowledge we acquire in science is additive. At its core is our ability to build on the knowledge of others. As scientists, we hope that others take note of what we have done, and use our work to go in directions we never imagined. In this way, we continue to add to our collective scientific legacy….” Nobel Lecture, 1997