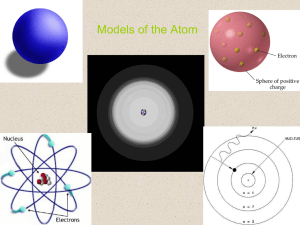
Models of the Atom
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
Inorganic Physical Methods
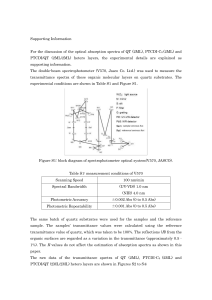
... Simplest way of recording a spectrum is to use a tunable monochromatic source. However, few of these can be tuned over a wide range of frequencies are available, so spectra are commonly recorded using a broad-band source, whose output contains all frequencies of interest. The problem then becomes ho ...
... Simplest way of recording a spectrum is to use a tunable monochromatic source. However, few of these can be tuned over a wide range of frequencies are available, so spectra are commonly recorded using a broad-band source, whose output contains all frequencies of interest. The problem then becomes ho ...
Synthesis of a New Structure B2H4 from B2H6 Highly Selective
... cleavage of a selected chemical bond in a complicated molecule remains a challenge in chemistry. Photochemists traditionally vary the ratios of cleavages of chemical bonds on tuning monochromatic radiation, typically in the ultraviolet region, to the energies of excited states of precursor molecules ...
... cleavage of a selected chemical bond in a complicated molecule remains a challenge in chemistry. Photochemists traditionally vary the ratios of cleavages of chemical bonds on tuning monochromatic radiation, typically in the ultraviolet region, to the energies of excited states of precursor molecules ...
Define:
... 95. What is the charge of a cation? An anion? 96. Cations form when an atom ______ _______ and anions form when an atom ______ _______. 97. Write the electron configuration for the sodium ion? Oxide ion? 98. How many electrons does strontium have to give up to achieve noble gas configuration? 99. Wh ...
... 95. What is the charge of a cation? An anion? 96. Cations form when an atom ______ _______ and anions form when an atom ______ _______. 97. Write the electron configuration for the sodium ion? Oxide ion? 98. How many electrons does strontium have to give up to achieve noble gas configuration? 99. Wh ...
IOSR Journal of Applied Physics (IOSR-JAP)
... mineral exploration and remote sensing(1,2). They are utilized in soil tests(3,4) and analysis for building constructions(5) and for agriculture. Atoms and molecules display different spectral types and patterns. Among these Raman spectroscopy plays a central role. Raman spectroscopy is one of the m ...
... mineral exploration and remote sensing(1,2). They are utilized in soil tests(3,4) and analysis for building constructions(5) and for agriculture. Atoms and molecules display different spectral types and patterns. Among these Raman spectroscopy plays a central role. Raman spectroscopy is one of the m ...
The Electromagnetic Spectrum: What`s the Use? Geology 1P Mr
... eye, but this energy exists at a wide range of wavelengths. The micron is the basic unit for measuring the wavelength of electromagnetic waves. The spectrum of waves is divided into sections based on wavelength. The shortest waves are gamma rays, which have wavelengths of 10e-6 microns or less. The ...
... eye, but this energy exists at a wide range of wavelengths. The micron is the basic unit for measuring the wavelength of electromagnetic waves. The spectrum of waves is divided into sections based on wavelength. The shortest waves are gamma rays, which have wavelengths of 10e-6 microns or less. The ...
The principles of transmission electron microscopy image formation
... value around 1 mm [6]. It means that electrons emitted in a cone with 1° half angle will be focused to a disc with 0.2 nm (2 Å) diameter. From other side, according to Abbe’s sine condition, resolution increases with increasing half-angle. In 1949, Scherzer found optimal values of resolution, focal ...
... value around 1 mm [6]. It means that electrons emitted in a cone with 1° half angle will be focused to a disc with 0.2 nm (2 Å) diameter. From other side, according to Abbe’s sine condition, resolution increases with increasing half-angle. In 1949, Scherzer found optimal values of resolution, focal ...
Sample Exam 3
... 17. You may have noticed that a bound electron (q = −e, m = me ) orbiting a proton (q = +e, m = mp ) in the Bohr model atom obeys the following relation: 2 KEn = –PEn . (a) If an excited electron orbits a proton at a distance of 1.9044 nm, what is the potential energy of this electron in eV? (b) Wha ...
... 17. You may have noticed that a bound electron (q = −e, m = me ) orbiting a proton (q = +e, m = mp ) in the Bohr model atom obeys the following relation: 2 KEn = –PEn . (a) If an excited electron orbits a proton at a distance of 1.9044 nm, what is the potential energy of this electron in eV? (b) Wha ...
Hands-on Activities with LEDs and Light
... • The normally empty conduction band of the semiconductor is populated by electrons injected into it by the forward current through the junction and light is generated when these electrons recombine with holes in the valence band to emit a photon. ...
... • The normally empty conduction band of the semiconductor is populated by electrons injected into it by the forward current through the junction and light is generated when these electrons recombine with holes in the valence band to emit a photon. ...
Supporting Information For the discussion of the optical absorption
... In contrast, the HOMO level is located around 5.9 eV, estimated by UPS measurements carried out by the authors [Figure S6] and Forker [18]. This result indicates that the ex-situ measurement reveals the QT HOMO level drops to a lower level or the optical band-gap expands after exposure to air. In an ...
... In contrast, the HOMO level is located around 5.9 eV, estimated by UPS measurements carried out by the authors [Figure S6] and Forker [18]. This result indicates that the ex-situ measurement reveals the QT HOMO level drops to a lower level or the optical band-gap expands after exposure to air. In an ...
Semester 1 Study Guide – Chemistry
... meaning that only certain discrete energy levels are allowed. ...
... meaning that only certain discrete energy levels are allowed. ...
Define:
... True or False: An ionic bond will form between a metal and a nonmetal and a covalent bond will form between nonmetals. ...
... True or False: An ionic bond will form between a metal and a nonmetal and a covalent bond will form between nonmetals. ...
Lecture 8: Nonclassical light • Squeezing • Photon anti
... in such a way that there are no simultaneous events at all. This is rather intuitive because there is one and only one photon present at any one time. Once that photon has been registered in one of the two detectors, it is destroyed and cannot appear at the other detector. A stream of light pulses e ...
... in such a way that there are no simultaneous events at all. This is rather intuitive because there is one and only one photon present at any one time. Once that photon has been registered in one of the two detectors, it is destroyed and cannot appear at the other detector. A stream of light pulses e ...
Notes - Particle Theory
... • Special relativity and quantum mechanics together imply the existence of anti-particles – special relativity treats negative and positive energies the same, implies the existence of an infinite tower of negative energy states (rather than a ground state) – we make sense of this by assuming all neg ...
... • Special relativity and quantum mechanics together imply the existence of anti-particles – special relativity treats negative and positive energies the same, implies the existence of an infinite tower of negative energy states (rather than a ground state) – we make sense of this by assuming all neg ...
Bohr`s equation for the hydrogen atom Bohr derived an equation to
... One result of this principle is that you can never squeeze two particles together to such an extent that they occupy the same state - objects must have a finite volume! It also means that if the exclusion priciple did not apply then all electrons in an atom would end up in the lowest possible energy ...
... One result of this principle is that you can never squeeze two particles together to such an extent that they occupy the same state - objects must have a finite volume! It also means that if the exclusion priciple did not apply then all electrons in an atom would end up in the lowest possible energy ...
Quantum Mechanical Model
... In all natural phenomena, change proceeds toward the lowest possible energy state. High energy systems are unstable. In atoms, the electrons and nucleus interact to make the most stable arrangement possible. The way in which they (electrons) are arranged around the nuclei of atoms is called electron ...
... In all natural phenomena, change proceeds toward the lowest possible energy state. High energy systems are unstable. In atoms, the electrons and nucleus interact to make the most stable arrangement possible. The way in which they (electrons) are arranged around the nuclei of atoms is called electron ...
Heisenberg: The Uncertainty Principle
... Measuring the position and momentum of an electron § By Planck’s law E = hc/λ, a photon with a short wavelength has a large energy § Thus, it would impart a large ‘kick’ to the electron § But to determine its momentum accurately, electron must only be given a small kick § This means using l ...
... Measuring the position and momentum of an electron § By Planck’s law E = hc/λ, a photon with a short wavelength has a large energy § Thus, it would impart a large ‘kick’ to the electron § But to determine its momentum accurately, electron must only be given a small kick § This means using l ...
Ch1 Mod Review.WXP
... interference pattern which is observed is not being produced at all points on the screen at the same time, but rather single points are darkened one at a time due to the interaction of the photon with the emulsion, and the location of individual points appears almost random. However, given enough ti ...
... interference pattern which is observed is not being produced at all points on the screen at the same time, but rather single points are darkened one at a time due to the interaction of the photon with the emulsion, and the location of individual points appears almost random. However, given enough ti ...
Unit 8: Electron Configuration
... field which allows the electrons which are like in charge (-) and would normally repel to attract. 4) Orbital - a region in space that can hold a maximum of 2 electrons with equal but opposite spins. Draw an s, p, d, and f orbital. ...
... field which allows the electrons which are like in charge (-) and would normally repel to attract. 4) Orbital - a region in space that can hold a maximum of 2 electrons with equal but opposite spins. Draw an s, p, d, and f orbital. ...
Solution set for the midterm exam
... of our instrument, be it the eye, or the microscope. Hence, we cannot resolve the graininess of the wavelength and the wave picture is obscured. For revealing the wave picture, one has to go to small momenta (mv) making the wavelength appreciably large so that our measuring apparatus can resolve the ...
... of our instrument, be it the eye, or the microscope. Hence, we cannot resolve the graininess of the wavelength and the wave picture is obscured. For revealing the wave picture, one has to go to small momenta (mv) making the wavelength appreciably large so that our measuring apparatus can resolve the ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.