electron cloud - Wickliffe City School
... The increased attraction pulls the cloud in, making atoms smaller as we move from left to right across a period. ...
... The increased attraction pulls the cloud in, making atoms smaller as we move from left to right across a period. ...
Chapter 4 4.1 Defining the Atom • Early Models of the Atom atom
... one element are different from those of any other element 3) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 4) Chemical reactions occur when atoms are separated from each other, joined, or rearranged in a different c ...
... one element are different from those of any other element 3) Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 4) Chemical reactions occur when atoms are separated from each other, joined, or rearranged in a different c ...
Atoms and Elements Notes
... • An ion is formed when an atom gives up an electron and becomes positive (+) or an atom gains an electron an becomes negative (–) • An ionic bond is formed when two opposite charged atoms come in contact and stick to together. • This the strongest of the chemical bonds ...
... • An ion is formed when an atom gives up an electron and becomes positive (+) or an atom gains an electron an becomes negative (–) • An ionic bond is formed when two opposite charged atoms come in contact and stick to together. • This the strongest of the chemical bonds ...
AP Chemistry
... 6.3.1 Monochromatic light = light of a single wavelength 6.3.2 Spectrum = when radiation from a source is separated into its different wavelengths 6.3.2.1 Continuous spectrum = rainbow of colors, containing light of all wavelengths 6.3.2.2 Some radiation sources give off light with only a few, speci ...
... 6.3.1 Monochromatic light = light of a single wavelength 6.3.2 Spectrum = when radiation from a source is separated into its different wavelengths 6.3.2.1 Continuous spectrum = rainbow of colors, containing light of all wavelengths 6.3.2.2 Some radiation sources give off light with only a few, speci ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... 'principle quantum number (n)' and 'effective nuclear charge (Zeff)'. How do these properties trend moving top to bottom and left to right on the Periodic Table? Make note of which property is most important in these two directions and how it changes on the diagram above. ...
... 'principle quantum number (n)' and 'effective nuclear charge (Zeff)'. How do these properties trend moving top to bottom and left to right on the Periodic Table? Make note of which property is most important in these two directions and how it changes on the diagram above. ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... 'principle quantum number (n)' and 'effective nuclear charge (Zeff)'. How do these properties trend moving top to bottom and left to right on the Periodic Table? Make note of which property is most important in these two directions and how it changes on the diagram above. ...
... 'principle quantum number (n)' and 'effective nuclear charge (Zeff)'. How do these properties trend moving top to bottom and left to right on the Periodic Table? Make note of which property is most important in these two directions and how it changes on the diagram above. ...
Document
... 2. EM radiation are forms of energy which move through space as waves a. Move at speed of light (c) 3.00 x 108 m/s b. Speed is equal to the frequency times the wavelength c = νλ Frequency (ν) (greek letter – nu) is the number of waves passing a given point in one second ...
... 2. EM radiation are forms of energy which move through space as waves a. Move at speed of light (c) 3.00 x 108 m/s b. Speed is equal to the frequency times the wavelength c = νλ Frequency (ν) (greek letter – nu) is the number of waves passing a given point in one second ...
The Quantum Mechanical Picture of the Atom
... Orbital The volume around the nucleus where the electron appears 90-95% of the time The Pauli principle No two electrons in an atom may have identical sets of four quantum numbers ...
... Orbital The volume around the nucleus where the electron appears 90-95% of the time The Pauli principle No two electrons in an atom may have identical sets of four quantum numbers ...
The energy
... frequency and energy value. » No value of E exceeds the ionization energy of Hydrogen – Eioniz = energy needed to remove 1 electron ...
... frequency and energy value. » No value of E exceeds the ionization energy of Hydrogen – Eioniz = energy needed to remove 1 electron ...
TANNIC ACID
... Consists of gallotannins obtained by solvent extraction from certain natural sources; the substance is not an acid in the chemical sense. The common name "Tannic acid" has been adopted to distinguish the commercial substance from other tannins, such as condensed tannins. These specifications relate ...
... Consists of gallotannins obtained by solvent extraction from certain natural sources; the substance is not an acid in the chemical sense. The common name "Tannic acid" has been adopted to distinguish the commercial substance from other tannins, such as condensed tannins. These specifications relate ...
What do you know about light?
... The chemical formula for water is always the same! – The composition of a molecule of water. The chemical formula tells us that a water molecule s made up of 3 atoms; 2 hydrogen and 1 oxygen. ...
... The chemical formula for water is always the same! – The composition of a molecule of water. The chemical formula tells us that a water molecule s made up of 3 atoms; 2 hydrogen and 1 oxygen. ...
Chemistry Chapter 5 Test Multiple Choice (1.5% each) Identify the
... 21. The region outside the nucleus where an electron can most probably be found is the a. electron cloud. c. quantum. b. s sublevel. 22. The distance between two successive peaks on a wave is its a. frequency. c. quantum number. b. wavelength. 23. The major difference between a 1s orbital and a 2s o ...
... 21. The region outside the nucleus where an electron can most probably be found is the a. electron cloud. c. quantum. b. s sublevel. 22. The distance between two successive peaks on a wave is its a. frequency. c. quantum number. b. wavelength. 23. The major difference between a 1s orbital and a 2s o ...
VSEPR Molecular Geometry VSEPR Molecular Geometry
... Determine central atom, eletropositive element Determine terminal/ peripheral atoms Connect central and terminal atoms Fulfill octet rule for terminal atoms Add electron to the central atom Determine the possibility of multiple bonds ...
... Determine central atom, eletropositive element Determine terminal/ peripheral atoms Connect central and terminal atoms Fulfill octet rule for terminal atoms Add electron to the central atom Determine the possibility of multiple bonds ...
IV. Single photon detection
... order to reduce the photon flux. This experimental set-up allows us to illuminate the junction with very few photons at each photodiode pulse by dilution of the optical fiber output beam. In addition, classical digital filtering methods are used to compute the photon counting data in order to minimi ...
... order to reduce the photon flux. This experimental set-up allows us to illuminate the junction with very few photons at each photodiode pulse by dilution of the optical fiber output beam. In addition, classical digital filtering methods are used to compute the photon counting data in order to minimi ...
Tabletop nanometer extreme ultraviolet imaging in an
... reconstruction, due to fewer overlapping scan positions in this region. With a larger scan, these artifacts will be completely eliminated for any structure of interest that is not near the edge of the scan range. Ptychography solves for the complex amplitudes of both the object and the probe (or inc ...
... reconstruction, due to fewer overlapping scan positions in this region. With a larger scan, these artifacts will be completely eliminated for any structure of interest that is not near the edge of the scan range. Ptychography solves for the complex amplitudes of both the object and the probe (or inc ...
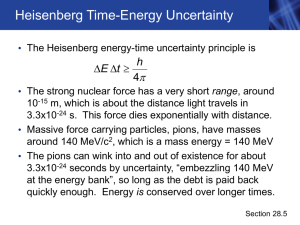
Chapter 28 - Purdue Physics
... how atoms are arranged on the surface • The tunneling current is highest when the tip is closest to an atom ...
... how atoms are arranged on the surface • The tunneling current is highest when the tip is closest to an atom ...
Analytical Chemistry/Pharmaceutical Analysis
... A qualitative analysis determines the presence or absence of a particular compound, but not the mass or concentration. That is, it is not related to quantity. Chemical tests There are numerous qualitative chemical tests, for example, the acid test for gold and the Kastle-Meyer test for the presence ...
... A qualitative analysis determines the presence or absence of a particular compound, but not the mass or concentration. That is, it is not related to quantity. Chemical tests There are numerous qualitative chemical tests, for example, the acid test for gold and the Kastle-Meyer test for the presence ...
ionization energies
... • A direct indication of the arrangement of electrons about a nucleus is given by the ionization energies of the atom • Ionization energy (IE) is the minimum energy needed to remove an electron (form a cation) completely from a gaseous atom • Ionizations are successive. • As you remove one electron, ...
... • A direct indication of the arrangement of electrons about a nucleus is given by the ionization energies of the atom • Ionization energy (IE) is the minimum energy needed to remove an electron (form a cation) completely from a gaseous atom • Ionizations are successive. • As you remove one electron, ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.