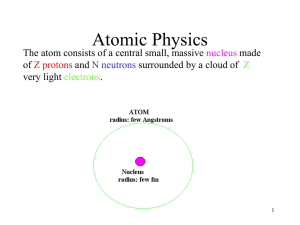
Document
... • Each of these states was associated with a fixed circular orbit of the electron around the nucleus. • Bohr proposed that atoms do not radiate energy while in one of their fixed (stationary) energy states. • When the electron moves to a different orbit, the atom changes to another energy state. • T ...
... • Each of these states was associated with a fixed circular orbit of the electron around the nucleus. • Bohr proposed that atoms do not radiate energy while in one of their fixed (stationary) energy states. • When the electron moves to a different orbit, the atom changes to another energy state. • T ...
Chapter 5 - Cloudfront.net
... • The atom evolved over the years as a result of new information that was collected. • The quantum mechanical model of the atom helped explain how electrons traveled around the nucleus and their energy. • Light and energy are related to each other and the movement of electrons. • Electrons are posit ...
... • The atom evolved over the years as a result of new information that was collected. • The quantum mechanical model of the atom helped explain how electrons traveled around the nucleus and their energy. • Light and energy are related to each other and the movement of electrons. • Electrons are posit ...
File
... orbital that can receive it. Pauli exclusion principle: no two electrons in the same atom can have the same set of four quantum numbers. Hund’s rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupi ...
... orbital that can receive it. Pauli exclusion principle: no two electrons in the same atom can have the same set of four quantum numbers. Hund’s rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupi ...
fulltext - DiVA portal
... The wave function contains all the dynamical information of the system it describes. Max Born interpreted Ψ(r) as a probability amplitude for some point r in space, and thus, the square of the wave function |Ψ(r)|2 is proportional to the probability of finding the particle at that point [6, 7]. This ...
... The wave function contains all the dynamical information of the system it describes. Max Born interpreted Ψ(r) as a probability amplitude for some point r in space, and thus, the square of the wave function |Ψ(r)|2 is proportional to the probability of finding the particle at that point [6, 7]. This ...
Classification – 3 main groups
... Atomic number identifies the element, is equal to the number of protons, if it changes you have a new element Isotope version of same element with different numbers of neutrons Mass number protons plus neutrons Ion a charged element due to the gain or loss of electrons Chapter 10 Lesson 1-3 Periodic ...
... Atomic number identifies the element, is equal to the number of protons, if it changes you have a new element Isotope version of same element with different numbers of neutrons Mass number protons plus neutrons Ion a charged element due to the gain or loss of electrons Chapter 10 Lesson 1-3 Periodic ...
teacher version filled in
... We know that the e-’s are free to move around the nucleus They also can move from one energy level to the next (and fall) back when energy is added ...
... We know that the e-’s are free to move around the nucleus They also can move from one energy level to the next (and fall) back when energy is added ...
Word - ASDL Community
... 1. What frequency of electromagnetic radiation is needed to excite a nuclear spin flip? 2. Where is radiofrequency (RF) radiation on the energy scale of the electromagnetic spectrum? 3. Is the thermal energy at room temperature large or small compared to the energy of a -* transition and to the en ...
... 1. What frequency of electromagnetic radiation is needed to excite a nuclear spin flip? 2. Where is radiofrequency (RF) radiation on the energy scale of the electromagnetic spectrum? 3. Is the thermal energy at room temperature large or small compared to the energy of a -* transition and to the en ...
Slides
... Adams and K.-J. Kim, to be published) The XFEL-O output pulses are copies of the same circulating intra-cavity pulse By stabilizing cavity RT time to less than 0.01l/c, the spectrum of XFELO output becomes a comb The extreme-stabilized XFEL-O will establish an x-ray-based length standard and h ...
... Adams and K.-J. Kim, to be published) The XFEL-O output pulses are copies of the same circulating intra-cavity pulse By stabilizing cavity RT time to less than 0.01l/c, the spectrum of XFELO output becomes a comb The extreme-stabilized XFEL-O will establish an x-ray-based length standard and h ...
Manipulating and Measuring the Quantum State of Photons and Atoms
... rough msmt of a given rate first and then deciding how long to acquire data on that point. (b) Could also measure populations first, and then avoid wasting time on coherences which would close to 0. (c) Even if r has only a few significant eigenvalues, is there a way to quickly figure out in which b ...
... rough msmt of a given rate first and then deciding how long to acquire data on that point. (b) Could also measure populations first, and then avoid wasting time on coherences which would close to 0. (c) Even if r has only a few significant eigenvalues, is there a way to quickly figure out in which b ...
Lectures 7-9 - U of L Class Index
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
Interaction of Photons with Matter
... If we excite atoms by bombardment with high energy electrons - say 10 keV or so - then as they de-excite the light they emit is of much higher frequency than the visible range. We call this light, of such high frequency - and hence low wavelength ( of order Angstroms) - that it can penetrate matter, ...
... If we excite atoms by bombardment with high energy electrons - say 10 keV or so - then as they de-excite the light they emit is of much higher frequency than the visible range. We call this light, of such high frequency - and hence low wavelength ( of order Angstroms) - that it can penetrate matter, ...
Lectures 7-9
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
... particles of matter can also behave as waves. Thus, his equation is not limited to electromagnetic radiation. In 1927, this was demonstrated by two separate experiments. Americans C.J. Davisson and L.H. Germer diffracted a beam of electrons through a nickel crystal, and Scot G.P. Thompson diffracted ...
Document
... Recoil energy increases internal kinetic energy of molecule in core ionized state. But core ionization takes maximum in turning point where kinetic energy is equal to zero This happens only if the transition is not vertical and it is shifted by: ...
... Recoil energy increases internal kinetic energy of molecule in core ionized state. But core ionization takes maximum in turning point where kinetic energy is equal to zero This happens only if the transition is not vertical and it is shifted by: ...
Quantum Physics in a Nutshell
... as a wave (but not a mechanical wave) • This theory states that electromagnetism behaves in a continuous way • Example: Your hand will get continuously warmer as it is near a hot object. • He also proposed that all electromagnetic waves traveled at c (in a vacuum). ...
... as a wave (but not a mechanical wave) • This theory states that electromagnetism behaves in a continuous way • Example: Your hand will get continuously warmer as it is near a hot object. • He also proposed that all electromagnetic waves traveled at c (in a vacuum). ...
extra information - Patrick Tevlin Music
... may or may not be more concentrated. This can be measured with a light meter. You would have to control for the flashlight beam’s greater area. A piece of cardboard with a hole the size of the laser beam might work. The best answer is C: The most significant difference between the two light sources ...
... may or may not be more concentrated. This can be measured with a light meter. You would have to control for the flashlight beam’s greater area. A piece of cardboard with a hole the size of the laser beam might work. The best answer is C: The most significant difference between the two light sources ...
Matter
... a material are dispersed throughout a liquid or gas but are large enough that they settle out. Particles are insoluble, so they DO NOT dissolve in the liquid or gas. Particles can be separated using a filter. ...
... a material are dispersed throughout a liquid or gas but are large enough that they settle out. Particles are insoluble, so they DO NOT dissolve in the liquid or gas. Particles can be separated using a filter. ...
Chemistry I Honors
... The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal energies Hybrid orbitals - equal energy produced by the combination of two or more orbitals on the same atom ...
... The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal energies Hybrid orbitals - equal energy produced by the combination of two or more orbitals on the same atom ...
Ch05ElectronConfig - Journigan-wiki
... could supply enough energy to eject an electron. Scientists could not explain why a certain minimum frequency was required. ...
... could supply enough energy to eject an electron. Scientists could not explain why a certain minimum frequency was required. ...
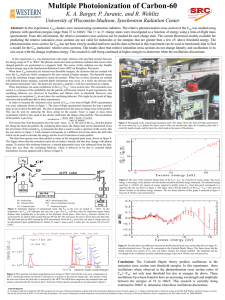
Figure 4 - University of Wisconsin–Madison
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.