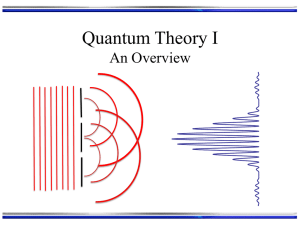
Slide 1
... excitation of electrons from ground state to excited state • Energy changes: 104 to 105 cm-1 or 100 to 1000 kJ mol-1 Four types of transitions: i) ...
... excitation of electrons from ground state to excited state • Energy changes: 104 to 105 cm-1 or 100 to 1000 kJ mol-1 Four types of transitions: i) ...
Chapter 5
... waves to one where our intuition will fail us. We can often get caught up in “That can’t be!” because we are use to thinking in a “classical” sense. Moore likes to use the word “quanton”, I find it a little cumbersome and I will use “quanta” (plural) or just “particle” but understand we mean somethi ...
... waves to one where our intuition will fail us. We can often get caught up in “That can’t be!” because we are use to thinking in a “classical” sense. Moore likes to use the word “quanton”, I find it a little cumbersome and I will use “quanta” (plural) or just “particle” but understand we mean somethi ...
Science, Systems, Matter, and Energy
... Heat from a stove burner causes atoms or molecules in the pan’s bottom to vibrate faster. The vibrating atoms or molecules then collide with nearby atoms or molecules, causing them to vibrate faster. Eventually, molecules or atoms in the pan’s handle are vibrating so fast it becomes too hot to touch ...
... Heat from a stove burner causes atoms or molecules in the pan’s bottom to vibrate faster. The vibrating atoms or molecules then collide with nearby atoms or molecules, causing them to vibrate faster. Eventually, molecules or atoms in the pan’s handle are vibrating so fast it becomes too hot to touch ...
Lecture 9
... Let’s do an experiment: Look at the discharge lamps through the diffraction glasses. They work just like a prism and break up light into its components. Notice the dark spots between the “lines” of the different colors. The number and positions of the lines are the unique signature of the elements. ...
... Let’s do an experiment: Look at the discharge lamps through the diffraction glasses. They work just like a prism and break up light into its components. Notice the dark spots between the “lines” of the different colors. The number and positions of the lines are the unique signature of the elements. ...
TAP 704- 7: Red shifts of quasars
... By what factor has the Universe expanded since light now reaching us left 3C273? ...
... By what factor has the Universe expanded since light now reaching us left 3C273? ...
2.8 Atomic Spectra of Hydrogen For some time scientist had known
... ordinary temperature, hydrogen atom, as well as most other atoms and molecules are found almost exclusively in their ground electronic states. The states of higher energy are called excited states and are generally unstable with respect to the ground state. An atom or a molecule in an excited state ...
... ordinary temperature, hydrogen atom, as well as most other atoms and molecules are found almost exclusively in their ground electronic states. The states of higher energy are called excited states and are generally unstable with respect to the ground state. An atom or a molecule in an excited state ...
Name: Date: Chemistry Enriched Per. ______ Midterm Review
... Atomic masses are not whole numbers due to isotopes, what are isotopes? ...
... Atomic masses are not whole numbers due to isotopes, what are isotopes? ...
"Particles or waves"()
... continuously through all possible energies until it comes to a halt. It does not slow down in discrete steps and there are no missing energies that are avoided as the speed decreases. Alternatively, imagine a red-hot poker cooling down. Since heat is a form of energy, the poker loses energy as it co ...
... continuously through all possible energies until it comes to a halt. It does not slow down in discrete steps and there are no missing energies that are avoided as the speed decreases. Alternatively, imagine a red-hot poker cooling down. Since heat is a form of energy, the poker loses energy as it co ...
Black body
... Introduction • The constant speed of light lead to Einstein’s special theory of relativity • We won’t need to use relativity for the spectroscopies we study ...
... Introduction • The constant speed of light lead to Einstein’s special theory of relativity • We won’t need to use relativity for the spectroscopies we study ...
Atomic Force Microscopy
... Tip vertically oscillates between contacting sample surface and lifting of at frequency of 50,000 to 500,000 cycles/sec. Oscillation amplitude reduced as probe contacts surface due to loss of energy caused by tip contacting surface Advantages: overcomes problems associated with friction, adhesio ...
... Tip vertically oscillates between contacting sample surface and lifting of at frequency of 50,000 to 500,000 cycles/sec. Oscillation amplitude reduced as probe contacts surface due to loss of energy caused by tip contacting surface Advantages: overcomes problems associated with friction, adhesio ...
Electronic Structure of Sr2RuO4
... Metallic Hydrogen Latest results: theory, again… • At very low temperatures, de Broglie wavelength becomes comparable to inter-atom separation • ! Metallic superfluid - or even a superconducting superfluid - at 4 Mbars? ...
... Metallic Hydrogen Latest results: theory, again… • At very low temperatures, de Broglie wavelength becomes comparable to inter-atom separation • ! Metallic superfluid - or even a superconducting superfluid - at 4 Mbars? ...
Quantum review
... Spectroscopic analysis of the visible spectrum… …produces all of the colors in a continuous spectrum ...
... Spectroscopic analysis of the visible spectrum… …produces all of the colors in a continuous spectrum ...
Abstract - University of Dayton
... in the liquid. These bubbles are characterized using the same probe and digital holography. An application of these bubbles to nanoparticle agglomeration and transport for drug delivery systems is proposed. Next, the use of recording materials such as photorefractive lithium niobate for implementing ...
... in the liquid. These bubbles are characterized using the same probe and digital holography. An application of these bubbles to nanoparticle agglomeration and transport for drug delivery systems is proposed. Next, the use of recording materials such as photorefractive lithium niobate for implementing ...
ppt
... sub-orbitals in the same energy level before a second can occupy the same sub-orbital Aufbau Principle: each electron is added to the lowest energy orbital available ...
... sub-orbitals in the same energy level before a second can occupy the same sub-orbital Aufbau Principle: each electron is added to the lowest energy orbital available ...
quantum mechanical model
... energy of atoms changes when the atom absorbs or emits light. • Stated that the electrons orbit the nucleus like planets orbit the Sun. ...
... energy of atoms changes when the atom absorbs or emits light. • Stated that the electrons orbit the nucleus like planets orbit the Sun. ...
Relativity - BrainMass
... 1. A 1.00-milliWatt laser produces photons of wavelength 633 nm. How many photons per second does the laser emit? 6.33 E+10 1.27 E+12 3.18 E+15 4.28 E+16 1.60 E+19 2. Monochromatic light shines on a surface with work function of 1.80 eV. The maximum kinetic energy of the emitted photoelectrons is 0. ...
... 1. A 1.00-milliWatt laser produces photons of wavelength 633 nm. How many photons per second does the laser emit? 6.33 E+10 1.27 E+12 3.18 E+15 4.28 E+16 1.60 E+19 2. Monochromatic light shines on a surface with work function of 1.80 eV. The maximum kinetic energy of the emitted photoelectrons is 0. ...
Chemistry Major Understandings
... 3.1f The mass of each proton and each neutron is approximately equal to one atomic mass unit. An electron is much less massive than a proton or a neutron. 3.1g The number of protons in an atom (atomic number) identifies the element. The sum of the protons and neutrons in an atom (mass number) identi ...
... 3.1f The mass of each proton and each neutron is approximately equal to one atomic mass unit. An electron is much less massive than a proton or a neutron. 3.1g The number of protons in an atom (atomic number) identifies the element. The sum of the protons and neutrons in an atom (mass number) identi ...
Nuclear Chemistry PowerPoint
... For example, the radioactive element bismuth (210Bi) can undergo alpha decay to form the element thallium (206Tl) with a reaction half-life equal to five days. • If we begin an experiment starting with 100 g of bismuth in a sealed lead container, after five days we will have 50 g of bismuth and 50 g ...
... For example, the radioactive element bismuth (210Bi) can undergo alpha decay to form the element thallium (206Tl) with a reaction half-life equal to five days. • If we begin an experiment starting with 100 g of bismuth in a sealed lead container, after five days we will have 50 g of bismuth and 50 g ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.