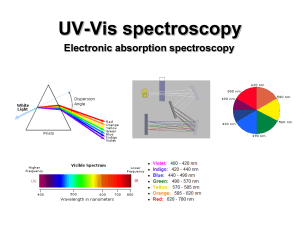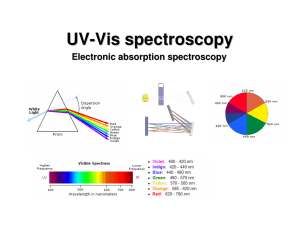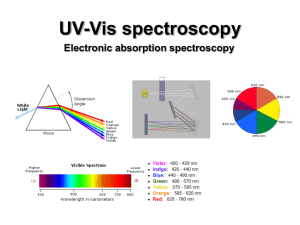
UV-Vis (electronic) spectroscopy
... Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
... Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
UV-Vis (electronic) spectroscopy
... Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
... Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
UV-Vis spectroscopy
... Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
... Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”. ...
Earth Science - Green Local Schools
... Know about sound waves … some specific characteristics that are unique to sound waves Look over the electromagnetic spectrum and wavelength…which has the greatest and smallest wavelengths? Which has the most/least energy? Look over reflection / refraction / diffraction charts Chapter 15 – Soun ...
... Know about sound waves … some specific characteristics that are unique to sound waves Look over the electromagnetic spectrum and wavelength…which has the greatest and smallest wavelengths? Which has the most/least energy? Look over reflection / refraction / diffraction charts Chapter 15 – Soun ...
Density Matrix
... Another concept that we shall use frequently is “energy level” or simply “level”. As you know, states of atoms or molecules in the gas phase almost always have degenerate magnetic substates as required by rotational invariance. The group of all such states of the same energy is called an energy leve ...
... Another concept that we shall use frequently is “energy level” or simply “level”. As you know, states of atoms or molecules in the gas phase almost always have degenerate magnetic substates as required by rotational invariance. The group of all such states of the same energy is called an energy leve ...
Intro to Optics - RosedaleGrade10Science
... Light is a visible form of energy, whose properties and behaviour can be modeled as waves.(Wave Model of Light) Light is a form of electromagnetic radiation, a wave pattern of electrical and magnetic fields that can travel through empty space. Other forms of electromagnetic radiation include invisib ...
... Light is a visible form of energy, whose properties and behaviour can be modeled as waves.(Wave Model of Light) Light is a form of electromagnetic radiation, a wave pattern of electrical and magnetic fields that can travel through empty space. Other forms of electromagnetic radiation include invisib ...
Lecture notes chapter 4
... Natural states of the elements: some elements consist of single atoms and they are found in an isolated state (for example, Ar and He). They are called monatomic elements. Some elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and ...
... Natural states of the elements: some elements consist of single atoms and they are found in an isolated state (for example, Ar and He). They are called monatomic elements. Some elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and ...
Building a Microwave Antenna for a Quantum Microscope
... When coupled, the pendulums will transfer energy. ...
... When coupled, the pendulums will transfer energy. ...
What is light? - Dipankar Home
... experiments is more intricate than Bohr envisaged. This provides further impetus to the effort initiated by several theorists, including the late John Bell, to contemplate something that has been almost unthinkable for 60 years - a fundamental revision of physicists' interpretation of what quantum m ...
... experiments is more intricate than Bohr envisaged. This provides further impetus to the effort initiated by several theorists, including the late John Bell, to contemplate something that has been almost unthinkable for 60 years - a fundamental revision of physicists' interpretation of what quantum m ...
Test Review: Unit 1 - Ms. Hill`s Pre
... a. Fusion: The combination of smaller molecule into larger ones. This happens on the sun. b. Fission: The splitting of large molecules into smaller radioactive daughter isotopes (“Mean Girls”) we do this in nuclear reactor and bombs! c. The big picture….both nuclear reaction result in the release of ...
... a. Fusion: The combination of smaller molecule into larger ones. This happens on the sun. b. Fission: The splitting of large molecules into smaller radioactive daughter isotopes (“Mean Girls”) we do this in nuclear reactor and bombs! c. The big picture….both nuclear reaction result in the release of ...
clicker questions 2
... Consider two light fields in vacuum, one at 532 nm (green), the other at 400 nm wavelength (blue). If you multiply the wavelength of each light field with the corresponding frequency , the result will be … (A) … a larger number for the green light field than for the blue ...
... Consider two light fields in vacuum, one at 532 nm (green), the other at 400 nm wavelength (blue). If you multiply the wavelength of each light field with the corresponding frequency , the result will be … (A) … a larger number for the green light field than for the blue ...
18. The Light Quantum Hypothesis.
... "Indeed, it seems to me that the observations of 'black-body radiation', photoluminescence, production of cathode rays by ultraviolet light, and other related phenomena associated with the emission or transformation of light appear more readily understood if one assumes that the energy of light is d ...
... "Indeed, it seems to me that the observations of 'black-body radiation', photoluminescence, production of cathode rays by ultraviolet light, and other related phenomena associated with the emission or transformation of light appear more readily understood if one assumes that the energy of light is d ...
The Photoelectric Effect
... the wavelength of light such that there was a sharp cut-off and no current flow for long wavelengths. Einstein successful explained the photoelectric effect within the context of the new physics of the time, quantum physics. In his scientific paper, he showed that light was made of packets of energy ...
... the wavelength of light such that there was a sharp cut-off and no current flow for long wavelengths. Einstein successful explained the photoelectric effect within the context of the new physics of the time, quantum physics. In his scientific paper, he showed that light was made of packets of energy ...
Electron Diffraction
... the intra-atomic distance in a crystal can be calculated by measuring the angle of electron diffraction and their wavelength (i.e. their momentum): d= ...
... the intra-atomic distance in a crystal can be calculated by measuring the angle of electron diffraction and their wavelength (i.e. their momentum): d= ...
Chemistry Curriculum Guide
... Ionization energy is the energy required to remove the most loosely held electron from a neutral atom. Elements with low ionization energy form positive ions (cations) easily. Elements with high ionization energy form negative ions (anions) easily. ...
... Ionization energy is the energy required to remove the most loosely held electron from a neutral atom. Elements with low ionization energy form positive ions (cations) easily. Elements with high ionization energy form negative ions (anions) easily. ...
Red Tide Specifications
... Specify standard or SAG+. Light enters the spectrometer, passes through the SMA Connector, Slit, and Filter, and then reflects off the Collimating Mirror onto the Grating. Diffracts light from the Collimating Mirror and directs the diffracted light onto the Focusing Mirror. Only Ocean Optics technic ...
... Specify standard or SAG+. Light enters the spectrometer, passes through the SMA Connector, Slit, and Filter, and then reflects off the Collimating Mirror onto the Grating. Diffracts light from the Collimating Mirror and directs the diffracted light onto the Focusing Mirror. Only Ocean Optics technic ...
Quantum Nonlinear Resonances in Atom Optics
... 1) Evaluation of the density of states and spectrum of the excitations 2) Quantization of the eigenmodes in the regime of stability: ...
... 1) Evaluation of the density of states and spectrum of the excitations 2) Quantization of the eigenmodes in the regime of stability: ...
Chemistry Midterm Review Study Guide 2012
... 10. If x is directly proportional to y, when x increases, y increases Sketch what this graph would look like below: ...
... 10. If x is directly proportional to y, when x increases, y increases Sketch what this graph would look like below: ...
... Entangled photon, a pair of simultaneous birth of twin photons, plays the heart of quantum information processing such as quantum teleportation, quantum cryptography and quantum computation. Its most amazing property is about simultaneous data transferring between two ports with which each entangled ...
Suman-AE-AOTFIntro-2..
... Where ∆n is the birefringence of the the TeO2 crystal, Vα and fα are the velocity and frequency of the acoustic wave, and α is a complex parameter depending on the design of the AOTF. The wavelength of the light that is selected by this diffraction can therefore be varied simply by changing the freq ...
... Where ∆n is the birefringence of the the TeO2 crystal, Vα and fα are the velocity and frequency of the acoustic wave, and α is a complex parameter depending on the design of the AOTF. The wavelength of the light that is selected by this diffraction can therefore be varied simply by changing the freq ...
Regents Chemistry Review Questions
... What is the chemical formula for ammonia? Is it an acid or a base? Write and balance the chemical equation for the neutralization reaction between carbonic acid and magnesium hydroxide. Name the salt that is produced in this reaction. Write and balance the chemical equation for the neutralization re ...
... What is the chemical formula for ammonia? Is it an acid or a base? Write and balance the chemical equation for the neutralization reaction between carbonic acid and magnesium hydroxide. Name the salt that is produced in this reaction. Write and balance the chemical equation for the neutralization re ...
chapter 7 quiz
... 15._P__The charge on an “gamma” particle. R) Henry Moseley 16._M__The empty space around the nucleus containing S) Dimitri Mendeleev electrons. T) atomic mass 17._Z__The name that describes protons, neutrons, U) chemical formula and electrons. V) proton 18._O__The short form way of representing an e ...
... 15._P__The charge on an “gamma” particle. R) Henry Moseley 16._M__The empty space around the nucleus containing S) Dimitri Mendeleev electrons. T) atomic mass 17._Z__The name that describes protons, neutrons, U) chemical formula and electrons. V) proton 18._O__The short form way of representing an e ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.