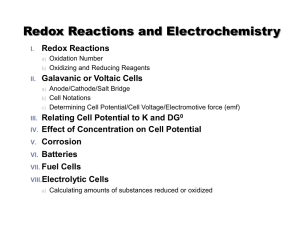
irreversible cell
... Single electrode potential (E): It is the measure of tendency of a metallic electrode to lose or gain electrons, when it is in contact with a solution of its own salt. Electrochemicall Cell: Electrochemical cell is the one, in which chemical energy is converted into electrical energy. The oxidation ...
... Single electrode potential (E): It is the measure of tendency of a metallic electrode to lose or gain electrons, when it is in contact with a solution of its own salt. Electrochemicall Cell: Electrochemical cell is the one, in which chemical energy is converted into electrical energy. The oxidation ...
Cell Function
... Many antibiotics work by inhibiting enzymes of disease-causing bacteria. Passive Transport: Diffusion across Membranes Molecules contain heat energy that causes them to vibrate and wander randomly. Diffusion is the tendency for molecules of any substance to spread out into the available space. Passi ...
... Many antibiotics work by inhibiting enzymes of disease-causing bacteria. Passive Transport: Diffusion across Membranes Molecules contain heat energy that causes them to vibrate and wander randomly. Diffusion is the tendency for molecules of any substance to spread out into the available space. Passi ...
1 - WordPress.com
... Science 9 Review Questions 8.2 Each electron is negative, all the same charge. If one is pushed by a negative voltage, it transmits a force across a distance to the next electron which transmits its force over a distance to the next etc. Thus the force exerted at one end is transmitted across a dis ...
... Science 9 Review Questions 8.2 Each electron is negative, all the same charge. If one is pushed by a negative voltage, it transmits a force across a distance to the next electron which transmits its force over a distance to the next etc. Thus the force exerted at one end is transmitted across a dis ...
General Chemistry 1412
... and uses of metal objects, however one technique to protecting metals from corrosion is to look at the standard electrode potentials and choose a more reactive metal to reverse the process and act as a sacrificial metal. ...
... and uses of metal objects, however one technique to protecting metals from corrosion is to look at the standard electrode potentials and choose a more reactive metal to reverse the process and act as a sacrificial metal. ...
REDOX PowerPoint - Southmoreland School District
... Pt (s) | H2 (1 atm) | H+ (1 M) || Cu2+ (1 M) | Cu (s) Anode (oxidation): ...
... Pt (s) | H2 (1 atm) | H+ (1 M) || Cu2+ (1 M) | Cu (s) Anode (oxidation): ...
Analytical animation-definitions electrochem3
... one can force the electron to go through a wire to do work, such as powering a light bulb. The device is called a galvanic cell. The electrodes are the two metal strips that dip into the solutions to conduct electricity. Very often they participate in the redox reactions. The cathode is where the re ...
... one can force the electron to go through a wire to do work, such as powering a light bulb. The device is called a galvanic cell. The electrodes are the two metal strips that dip into the solutions to conduct electricity. Very often they participate in the redox reactions. The cathode is where the re ...
112 ex i lec outline
... Since an electrode potential, E°, depends upon the concentration of the solutions used in the electrode, a cell may be constructed from two half-cells composed of the same materials but differing in concentration of ions. The spontaneous reaction occurs in the directions that tends to make the two i ...
... Since an electrode potential, E°, depends upon the concentration of the solutions used in the electrode, a cell may be constructed from two half-cells composed of the same materials but differing in concentration of ions. The spontaneous reaction occurs in the directions that tends to make the two i ...
Controlling many-body states by the electric-field effect in a
... theory, many different electronic states could be observed in a single sample if the electron behavior could be controlled externally. By simply modifying the number of electrons, or carrier concentration, the material switches between different electronic states, including metals, semiconductors, e ...
... theory, many different electronic states could be observed in a single sample if the electron behavior could be controlled externally. By simply modifying the number of electrons, or carrier concentration, the material switches between different electronic states, including metals, semiconductors, e ...
Electrochemistry 2
... but double the current can flow before the cells run out of one of the metals. ...
... but double the current can flow before the cells run out of one of the metals. ...
Unit Powerpoint
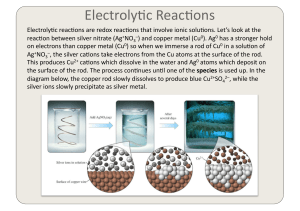
... into an ion with a 2+ charge. This happens when it loses 2 electrons. Cu (s) Cu2+ (aq) + 2 eCopper was oxidized because it lost electrons. Silver went from an ion Ag+ to a neutral atom Ag. The only way this can happen is to gain electrons. It has been reduced. ...
... into an ion with a 2+ charge. This happens when it loses 2 electrons. Cu (s) Cu2+ (aq) + 2 eCopper was oxidized because it lost electrons. Silver went from an ion Ag+ to a neutral atom Ag. The only way this can happen is to gain electrons. It has been reduced. ...
Text S1.
... removed by centrifugation at 1,000 x G. The resulting supernatants were centrifuged again at 10,000 x G to remove cytosolic fractions. The pellets were washed once with Buffer C and used as mitochondria enriched fractions. To obtain integral membrane protein, mitochondria enriched fractions were sub ...
... removed by centrifugation at 1,000 x G. The resulting supernatants were centrifuged again at 10,000 x G to remove cytosolic fractions. The pellets were washed once with Buffer C and used as mitochondria enriched fractions. To obtain integral membrane protein, mitochondria enriched fractions were sub ...
AP Ch. 20 Notes (2005)
... Zn is spontaneously oxidized into Zn2+ by the Cu2+. The Cu2+ is spontaneously reduced into Cu0 by the Zn. The entire process is spontaneous! ...
... Zn is spontaneously oxidized into Zn2+ by the Cu2+. The Cu2+ is spontaneously reduced into Cu0 by the Zn. The entire process is spontaneous! ...
Chemical Thermodynamics -
... Two Types electrolytic cell converts electrical energy into chemical energy electricity is used to drive a non-spontaneous reaction galvanic (or voltaic) cell converts chemical energy into electricity (a battery!) a spontaneous reaction produces electricity Conduction metals ...
... Two Types electrolytic cell converts electrical energy into chemical energy electricity is used to drive a non-spontaneous reaction galvanic (or voltaic) cell converts chemical energy into electricity (a battery!) a spontaneous reaction produces electricity Conduction metals ...
Document
... Two Types electrolytic cell converts electrical energy into chemical energy electricity is used to drive a non-spontaneous reaction galvanic (or voltaic) cell converts chemical energy into electricity (a battery!) a spontaneous reaction produces electricity Conduction metals ...
... Two Types electrolytic cell converts electrical energy into chemical energy electricity is used to drive a non-spontaneous reaction galvanic (or voltaic) cell converts chemical energy into electricity (a battery!) a spontaneous reaction produces electricity Conduction metals ...
Factors affecting microbial transport in soil
... Be able to list and understand the four factors that affect microbial transport ...
... Be able to list and understand the four factors that affect microbial transport ...
Lecture notes on the discovery of the electron
... matter–that is, matter derived from different sources such as hydrogen, oxygen, etc.–is of one and the same kind; this matter being the substance from which the chemical elements are built up.” He had leaped to the conclusion that the particles in the cathode ray (which we now call electrons) were a ...
... matter–that is, matter derived from different sources such as hydrogen, oxygen, etc.–is of one and the same kind; this matter being the substance from which the chemical elements are built up.” He had leaped to the conclusion that the particles in the cathode ray (which we now call electrons) were a ...
Oxidation and Reduction Reactions
... reactivity series for metals and halogens – what do they show? predicting relative strengths of oxidizing and reducing agents from a reactivity series predicting whether or not a reaction occurs based on the reactivity series constructing a reactivity series based on experimental observations of rea ...
... reactivity series for metals and halogens – what do they show? predicting relative strengths of oxidizing and reducing agents from a reactivity series predicting whether or not a reaction occurs based on the reactivity series constructing a reactivity series based on experimental observations of rea ...
1 7 – Electrochemical conversion 1. Introduction Some successive
... released heat. Even a global endothermic reaction was realized with heat consumption from an external source. If that heat quantity originates as residual heat of other processes, the global conversion efficiency will be higher. Such a secondary source may be the cooling water of a power station. Fu ...
... released heat. Even a global endothermic reaction was realized with heat consumption from an external source. If that heat quantity originates as residual heat of other processes, the global conversion efficiency will be higher. Such a secondary source may be the cooling water of a power station. Fu ...
electrochemical cell
... if two substances have a great difference in their tendency of lose and acquire electrons, the voltage of the cell will be high ; cell voltage can be used to predict whether a particular redox reaction will occur spontaneously ; ...
... if two substances have a great difference in their tendency of lose and acquire electrons, the voltage of the cell will be high ; cell voltage can be used to predict whether a particular redox reaction will occur spontaneously ; ...
Electrochemistry File
... The S.H.E. is not a convenient electrode for regular use as a reference. A reference electrode needs to be easy to use, stable, reproducible, and reliable. The calomel electrode is the electrode of choice: Pt (s) | Hg (l) | Hg2Cl2 (s) | saturated ...
... The S.H.E. is not a convenient electrode for regular use as a reference. A reference electrode needs to be easy to use, stable, reproducible, and reliable. The calomel electrode is the electrode of choice: Pt (s) | Hg (l) | Hg2Cl2 (s) | saturated ...
Electrolysis, the Faraday, and Avogadro`s Number
... Electrolysis, the Faraday, and Avogadro’s Number ...
... Electrolysis, the Faraday, and Avogadro’s Number ...
Chapter 18 Electrochemistry
... Electrochemical processes are oxidation-reduction reactions in which: ...
... Electrochemical processes are oxidation-reduction reactions in which: ...
gallagher chapter 21 08
... • Chemical processes either release or absorb energy – Energy is sometimes in the form of electricity ...
... • Chemical processes either release or absorb energy – Energy is sometimes in the form of electricity ...
Redox Reactions
... 15e4e4FeS2 + 15O2 Fe(OH)3 + SO424FeS2 + 15O2 4Fe(OH)3 + 8SO424FeS2 + 15O2 +14H2O 4Fe(OH)3 + 8SO42- + 16H+ This reaction is the main cause of acid generation in drainage from sulfide ore deposits. Note that we get 4 moles of H+ for every mole of pyrite oxidized! ...
... 15e4e4FeS2 + 15O2 Fe(OH)3 + SO424FeS2 + 15O2 4Fe(OH)3 + 8SO424FeS2 + 15O2 +14H2O 4Fe(OH)3 + 8SO42- + 16H+ This reaction is the main cause of acid generation in drainage from sulfide ore deposits. Note that we get 4 moles of H+ for every mole of pyrite oxidized! ...