Chemistry Handout 08 - (Redox)
... According to the Activity Series chemistry reference table, which of these metals will react most readily with 1.0 M HCl to produce H2 (g)? A) Zn ...
... According to the Activity Series chemistry reference table, which of these metals will react most readily with 1.0 M HCl to produce H2 (g)? A) Zn ...
and their direct dependency upon fluid
... Dissolved Oxygen now below detection Now target anaerobic enrichments: Isolates for biochemical and proteomic studies Anaerobic Fe-oxidizers Mn-reducers Fe-reducers Sulfate-reducers ...
... Dissolved Oxygen now below detection Now target anaerobic enrichments: Isolates for biochemical and proteomic studies Anaerobic Fe-oxidizers Mn-reducers Fe-reducers Sulfate-reducers ...
Lectures 27-30 - U of L Class Index
... An lead-acid battery has a lead anode and a lead(IV) oxide cathode. As the name implies, it operates under acidic conditions (HSO4-(aq)): ...
... An lead-acid battery has a lead anode and a lead(IV) oxide cathode. As the name implies, it operates under acidic conditions (HSO4-(aq)): ...
Lectures 26-28
... An lead-acid battery has a lead anode and a lead(IV) oxide cathode. As the name implies, it operates under acidic conditions (HSO4-(aq)): ...
... An lead-acid battery has a lead anode and a lead(IV) oxide cathode. As the name implies, it operates under acidic conditions (HSO4-(aq)): ...
Lectures 28-31 - U of L Class Index
... An lead-acid battery has a lead anode and a lead(IV) oxide cathode. As the name implies, it operates under acidic conditions (HSO4-(aq)): ...
... An lead-acid battery has a lead anode and a lead(IV) oxide cathode. As the name implies, it operates under acidic conditions (HSO4-(aq)): ...
Document
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
(.pdf format)
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Electrochemistry
... Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur. Electrolysis of molten NaCl ...
... Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur. Electrolysis of molten NaCl ...
Document
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Chem163_Electrochem
... the oxidation and reduction half-reaction are placed in separate compartments called half-cells. The metal of each half-reaction is called an electrode, and is placed in the solution and connected to an external wire. The electrode at which oxidation occurs is called the anode and the electrode at w ...
... the oxidation and reduction half-reaction are placed in separate compartments called half-cells. The metal of each half-reaction is called an electrode, and is placed in the solution and connected to an external wire. The electrode at which oxidation occurs is called the anode and the electrode at w ...
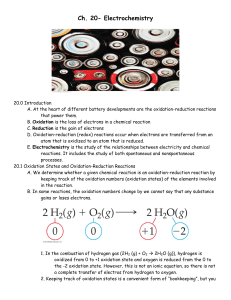
Ch. 20- Electrochemistry
... 1. Rusting of iron requires both oxygen and water, and the process can be accelerated by other factors such as pH, presence of salts, contact with metals more difficult to oxidize than iron, and stress on the iron. E. Preventing Corrosion of Iron 1. Objects made of iron are often covered with a coat ...
... 1. Rusting of iron requires both oxygen and water, and the process can be accelerated by other factors such as pH, presence of salts, contact with metals more difficult to oxidize than iron, and stress on the iron. E. Preventing Corrosion of Iron 1. Objects made of iron are often covered with a coat ...
Document
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Expriment5-labReport-Spring2017
... 4. Calculate the maximum electric work (in kJ/mol of H2) using an experimentally measured open circuit cell potential from the previous table assuming that only the reversible electrical work is performed at constant temperature and pressure. Compare this with the Wel max calculated under standard c ...
... 4. Calculate the maximum electric work (in kJ/mol of H2) using an experimentally measured open circuit cell potential from the previous table assuming that only the reversible electrical work is performed at constant temperature and pressure. Compare this with the Wel max calculated under standard c ...
experiment 7 - (canvas.brown.edu).
... reactions and calculate the standard fuel cell potential, Eo (or open circuit potential), using their standard half cell reduction potentials (See Zumdahl Appendix five, or Tro Appendix II D). ...
... reactions and calculate the standard fuel cell potential, Eo (or open circuit potential), using their standard half cell reduction potentials (See Zumdahl Appendix five, or Tro Appendix II D). ...
Gordon Chua: Biographical Abstract
... Advanced Education and the Canadian Foundation for Innovation. This research program particularly targets bacteria with applications to energy and climate issues, such as the reduction of methane emissions and the production of sustainable biofuels. For example, they are studying soil bacteria that ...
... Advanced Education and the Canadian Foundation for Innovation. This research program particularly targets bacteria with applications to energy and climate issues, such as the reduction of methane emissions and the production of sustainable biofuels. For example, they are studying soil bacteria that ...
E 0
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Chapter 20b - U of L Class Index
... Note that, in most commercial voltaic cells, pastes of inorganic salts are used. This allows compact storage of the salts. The water in the paste dissolves some salt to make a saturated solution in which the ion concentrations do not significantly decrease until near the end of the cell’s usefulness ...
... Note that, in most commercial voltaic cells, pastes of inorganic salts are used. This allows compact storage of the salts. The water in the paste dissolves some salt to make a saturated solution in which the ion concentrations do not significantly decrease until near the end of the cell’s usefulness ...
Ch. 20 study questions
... A copper-zinc voltaic cell is constructed using100 mL solutions of 1M solutions of copper sulfate and zinc sulfate with a sodium sulfate salt bridge. After some time, t, had passed at 25C, the concentration of the Zn2+ ions in the anode half cell had increased to 1.50M and the concentration of the ...
... A copper-zinc voltaic cell is constructed using100 mL solutions of 1M solutions of copper sulfate and zinc sulfate with a sodium sulfate salt bridge. After some time, t, had passed at 25C, the concentration of the Zn2+ ions in the anode half cell had increased to 1.50M and the concentration of the ...
Ch 20 Electrochemistry:
... 1. All potentials are for reduction reactions. 2. The more positive E°, the better the oxidizing ability. 3. The more negative E°, the more likely the reverse (oxidation) reaction will occur. 4. When the reaction is reversed, the sign changes. 5. NW-SE rule: reaction of the upper left with the lower ...
... 1. All potentials are for reduction reactions. 2. The more positive E°, the better the oxidizing ability. 3. The more negative E°, the more likely the reverse (oxidation) reaction will occur. 4. When the reaction is reversed, the sign changes. 5. NW-SE rule: reaction of the upper left with the lower ...
Review redox reactions
... side to “neutralize” the H+ in the equation and create water in its place. If this produces water on both sides, you might have to subtract water from each side. ...
... side to “neutralize” the H+ in the equation and create water in its place. If this produces water on both sides, you might have to subtract water from each side. ...
Tutorial 5 - Electrochemistry
... Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) Cell potentials The cell potential, E, is a measure of how well a cell reaction can push and pull electrons through a circuit ...
... Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) Cell potentials The cell potential, E, is a measure of how well a cell reaction can push and pull electrons through a circuit ...
Student Notes
... • There are environmental concerns to be addressed regarding the disposal of such batteries. • Other rechargeable batteries have been developed. • NiMH batteries (nickel-metal-hydride). • Li-ion batteries (lithium-ion batteries). Hydrogen Fuel Cells • Direct production of electricity from fuels occu ...
... • There are environmental concerns to be addressed regarding the disposal of such batteries. • Other rechargeable batteries have been developed. • NiMH batteries (nickel-metal-hydride). • Li-ion batteries (lithium-ion batteries). Hydrogen Fuel Cells • Direct production of electricity from fuels occu ...
Oxidation number and Electrolysis(電解)
... concentrated NaCl solution, only H + is discharged at the cathode. But if mercury electrode is used for the cathode, Na + is discharged because sodium metal forms an alloy with mercury. (This method is used in industry for the production of sodium.) ...
... concentrated NaCl solution, only H + is discharged at the cathode. But if mercury electrode is used for the cathode, Na + is discharged because sodium metal forms an alloy with mercury. (This method is used in industry for the production of sodium.) ...
Fuel Cells – an Introduction
... If you remove the external voltage (battery) from the electrolysis experiment, the rising gas bubbles stop but many of them are left sticking to the electrodes. An electric voltage will still be measured on such a cell even after the external voltage is ...
... If you remove the external voltage (battery) from the electrolysis experiment, the rising gas bubbles stop but many of them are left sticking to the electrodes. An electric voltage will still be measured on such a cell even after the external voltage is ...