Guide for Cell Biology
... * Identifying substances such as gelatin, vitamin C, glucose, butter, and cornstarch using reagent tests. * Identifying key organic chemicals and their role in cell operation. PHOTOSYNTHESIS AND CELLULAR RESPIRATION * Calculating the energy content of food from data obtained from calorimeters. Relat ...
... * Identifying substances such as gelatin, vitamin C, glucose, butter, and cornstarch using reagent tests. * Identifying key organic chemicals and their role in cell operation. PHOTOSYNTHESIS AND CELLULAR RESPIRATION * Calculating the energy content of food from data obtained from calorimeters. Relat ...
Electrochemistry - Northwest ISD Moodle
... is spontaneous. A solution containing K2Cr2O7 and H2SO4 is poured into one beaker, and a solution of KI is poured into another. A salt bridge is used to join the beakers. A metallic conductor that will not react with either solution (such as platinum foil) is suspended in each solution, and the two ...
... is spontaneous. A solution containing K2Cr2O7 and H2SO4 is poured into one beaker, and a solution of KI is poured into another. A salt bridge is used to join the beakers. A metallic conductor that will not react with either solution (such as platinum foil) is suspended in each solution, and the two ...
Topic 9 - uaschemistry
... • 9.4.2 State that oxidation occurs at the negative electrode (anode) and reduction occurs at the positive electrode (cathode). • State: Give a specific name, value or other brief answer without explanation or ...
... • 9.4.2 State that oxidation occurs at the negative electrode (anode) and reduction occurs at the positive electrode (cathode). • State: Give a specific name, value or other brief answer without explanation or ...
Chapter 18 - WordPress.com
... Many times the anode is made of the metal that is oxidized and the cathode is made of the same metal as is produced by the reduction. However, if the redox reaction we are running involves the oxidation or reduction of an ion to a different oxidation state, or the oxidation or reduction of a gas, we ...
... Many times the anode is made of the metal that is oxidized and the cathode is made of the same metal as is produced by the reduction. However, if the redox reaction we are running involves the oxidation or reduction of an ion to a different oxidation state, or the oxidation or reduction of a gas, we ...
Text S1, DOCX file, 0.1 MB
... the public American Gut repository (ftp.microbio.me/AmericanGut/rounds-1-25/). Following the method described by the online analytical methods on the American Gut cohort, we filtered the cohort to conserve samples based on the following criteria: adults 20-69 years of age with BMIs ranging between 1 ...
... the public American Gut repository (ftp.microbio.me/AmericanGut/rounds-1-25/). Following the method described by the online analytical methods on the American Gut cohort, we filtered the cohort to conserve samples based on the following criteria: adults 20-69 years of age with BMIs ranging between 1 ...
Chapter 15 Notes - Mr. Julien`s Homepage
... Cu(s) (reduction) c. The overall cell reaction is: Zn(s) + Cu2+(aq) Cu(s) + Zn2+(aq) 4. The transfer of electrons is direct from Zn to Cu2+ but the reaction can be divided into half-cells. 5. Electrons flow from one half-cell to the other when an external circuit connects half-cells. a. Anode— b. ...
... Cu(s) (reduction) c. The overall cell reaction is: Zn(s) + Cu2+(aq) Cu(s) + Zn2+(aq) 4. The transfer of electrons is direct from Zn to Cu2+ but the reaction can be divided into half-cells. 5. Electrons flow from one half-cell to the other when an external circuit connects half-cells. a. Anode— b. ...
CHEMISTRY 1.2 LECTURE
... Change of Enthalpy,H, is the heat given off or absorbed by a system at constant pressure. For reactions, the change of enthalpy is the Heat of Reaction: Heat of Reaction = Hrxn = Hfinal – Hinitial and Hrxn = q ...
... Change of Enthalpy,H, is the heat given off or absorbed by a system at constant pressure. For reactions, the change of enthalpy is the Heat of Reaction: Heat of Reaction = Hrxn = Hfinal – Hinitial and Hrxn = q ...
SOFCs. Electrolytes
... - Small to average size cogeneration systems (hot water + electricity). •Large scale power generation and car engines: no longer a target. •Advantages: better conversion efficiency (60%; >90% in cogeneration) in comparison to combustion engines and gas turbines (25%): lower environmental impact. Ste ...
... - Small to average size cogeneration systems (hot water + electricity). •Large scale power generation and car engines: no longer a target. •Advantages: better conversion efficiency (60%; >90% in cogeneration) in comparison to combustion engines and gas turbines (25%): lower environmental impact. Ste ...
Chapter 20 - public.asu.edu
... Balance by the same procedure. Equalize the number of electrons lost and gained by multiplying each coefficient in each half-reaction by the appropriate constant. Add the two half-reactions and cancel equal amounts of anything occurring on both sides. Make a final check of atom and charge balances. ...
... Balance by the same procedure. Equalize the number of electrons lost and gained by multiplying each coefficient in each half-reaction by the appropriate constant. Add the two half-reactions and cancel equal amounts of anything occurring on both sides. Make a final check of atom and charge balances. ...
Franck-Hertz Effect in Mercury
... The anode is a perforated screen, so many electrons will pass through. They can proceed to the collector, if their kinetic energy at the anode is greater than eVretarding, where Vret is the small, adjustable voltage between anode and collector. Otherwise, they slow, stop, and reverse direction to re ...
... The anode is a perforated screen, so many electrons will pass through. They can proceed to the collector, if their kinetic energy at the anode is greater than eVretarding, where Vret is the small, adjustable voltage between anode and collector. Otherwise, they slow, stop, and reverse direction to re ...
Schmidt, C., Behrens, S., Kappler, A. (2010)
... (i.e. light restriction, substrate delivery) or generally the lack of a specific substrate (i.e. nitrate-depleted environments)), the microbial arrangement might deviate from the above suggested scenario. In environments that lack nitrate, the boundaries and the predominant spatial location of photo ...
... (i.e. light restriction, substrate delivery) or generally the lack of a specific substrate (i.e. nitrate-depleted environments)), the microbial arrangement might deviate from the above suggested scenario. In environments that lack nitrate, the boundaries and the predominant spatial location of photo ...
X-Ray Production
... for tungsten 59 keV corresponds to the difference in energy between K and L ...
... for tungsten 59 keV corresponds to the difference in energy between K and L ...
Red-ox reactions Electochemistry
... A rechargeable battery, storage battery, or accumulator is a type of electrical battery. It comprises one or more electrical cells, and is a type of energy accumulator. It is known as a secondary cell because its electrochemical reactions are ...
... A rechargeable battery, storage battery, or accumulator is a type of electrical battery. It comprises one or more electrical cells, and is a type of energy accumulator. It is known as a secondary cell because its electrochemical reactions are ...
Red-ox reactions Electochemistry
... A rechargeable battery, storage battery, or accumulator is a type of electrical battery. It comprises one or more electrical cells, and is a type of energy accumulator. It is known as a secondary cell because its electrochemical reactions are ...
... A rechargeable battery, storage battery, or accumulator is a type of electrical battery. It comprises one or more electrical cells, and is a type of energy accumulator. It is known as a secondary cell because its electrochemical reactions are ...
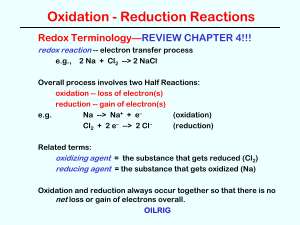
Oxidation and Reduction Reactions
... It dissolves into solution as metal strip gets OXIDIZED. It is the ANODE This replenishes the ions for ...
... It dissolves into solution as metal strip gets OXIDIZED. It is the ANODE This replenishes the ions for ...
Electrochemistry
... and charges. (Look at medium) Step 4: Add the two half-reactions together and balance the final equation by inspection. The electrons on both sides must cancel. (Be sure they are equal) Step 5: Verify that the equation contains the same type and numbers of atoms and the same charges on both sides of ...
... and charges. (Look at medium) Step 4: Add the two half-reactions together and balance the final equation by inspection. The electrons on both sides must cancel. (Be sure they are equal) Step 5: Verify that the equation contains the same type and numbers of atoms and the same charges on both sides of ...
서울대학교 일반화학실험
... electromagentic induction was discovered by Faraday, electrochemical cell had been discovered by Galvani and Volta. And electrochemical cell had been used in electrolysis of water and in the discovery of several metallic elements prominently by Humphrey Davy. The driving force of an electrochemical ...
... electromagentic induction was discovered by Faraday, electrochemical cell had been discovered by Galvani and Volta. And electrochemical cell had been used in electrolysis of water and in the discovery of several metallic elements prominently by Humphrey Davy. The driving force of an electrochemical ...
CHEMISTRY 112 LECTURE
... II. State Functions State Functions are properties that on dependent upon its present state and not dependent upon the pathway to the present state. For example: the Internal energy (E) of a system is a state function = its value depends only on the state of the system and not how it arrived at that ...
... II. State Functions State Functions are properties that on dependent upon its present state and not dependent upon the pathway to the present state. For example: the Internal energy (E) of a system is a state function = its value depends only on the state of the system and not how it arrived at that ...
1. A [1] 2. B [1] 3. Dilute sodium chloride: 2H 2 O → O 2 + 4H + + 4e
... Do not award M3 for “metal deposited at cathode where oxidation occurs”. (ii) ...
... Do not award M3 for “metal deposited at cathode where oxidation occurs”. (ii) ...
Electron notes File
... D orbital 10 electrons • 4th energy level - S orbital 2 electrons P orbital 6 electrons D orbital 10 electrons F orbital 14 electrons ...
... D orbital 10 electrons • 4th energy level - S orbital 2 electrons P orbital 6 electrons D orbital 10 electrons F orbital 14 electrons ...
FUEL CELL
... Hydrogen is odourless, colourless, tasteless and nontoxic. Hydrogen has a very wide range of flammability. Hydrogen is very buoyant and diffuses rapidly in air. Hydrogen has very low ignition energy. Hydrogen burns with a pale blue, nearly invisible, flame. Hydrogen is non-toxic and non-poisonous. H ...
... Hydrogen is odourless, colourless, tasteless and nontoxic. Hydrogen has a very wide range of flammability. Hydrogen is very buoyant and diffuses rapidly in air. Hydrogen has very low ignition energy. Hydrogen burns with a pale blue, nearly invisible, flame. Hydrogen is non-toxic and non-poisonous. H ...
Corrosion - iMechanica
... When a metal is exposed to an aqueous environment it develops an electric potential called a half-cell potential that is characteristic of the metal and the environment. This potential can be measured by using a reference electrode. Table 1 lists the half-cell potentials under standard conditions, f ...
... When a metal is exposed to an aqueous environment it develops an electric potential called a half-cell potential that is characteristic of the metal and the environment. This potential can be measured by using a reference electrode. Table 1 lists the half-cell potentials under standard conditions, f ...


















![1. A [1] 2. B [1] 3. Dilute sodium chloride: 2H 2 O → O 2 + 4H + + 4e](http://s1.studyres.com/store/data/011637084_1-98d57769d10f8d697c9e5678e340d457-300x300.png)