* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download SOFCs. Electrolytes
Surface properties of transition metal oxides wikipedia , lookup
Transition state theory wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Heat transfer physics wikipedia , lookup
History of electrochemistry wikipedia , lookup
Microbial fuel cell wikipedia , lookup
Microplasma wikipedia , lookup
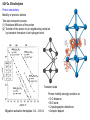
Ionic ceramic conductors. Solid Oxide Fuell Cells (SOFCs) Fuel cells. Generalities Fuel cells (FCs): electrochemical devices for the direct conversion of chemical energy in electricity by redox reactions at the electrodes. Differently from batteries, FCs are open systems wich allow continuous supply of the reactants (oxygen/air at cathode, hydrogen/hydrocarbons at anode). •First application: power generation in space (Gemini & Apollo missions). •Current applications (still under development): - Miniaturized power generation for portable electronic devices (notebooks, tablets, mobile phones, military applications.) - Small to average size cogeneration systems (hot water + electricity). •Large scale power generation and car engines: no longer a target. •Advantages: better conversion efficiency (60%; >90% in cogeneration) in comparison to combustion engines and gas turbines (25%): lower environmental impact. Steady power. •Fully clean energy production using H2 as fuel: still a dream. •Drawbacks: still suffer of reliability issues and short operation time (target: 40000 h/5y). Replacement of combustion engines requires hybrid electrochemical devices Fuel cells. Existing technologies Alkaline Polymeric electrolyte membrane Direct methanol Phosphoric acid Molten carbonate Solid oxide Anode: negative electrode associated with fuel (H2) oxidation and release of electrons into the external circuit (porous). Cathode: positive electrode associated with reduction of the oxidant (O2) that gains electrons from the external circuit (porous). Electrolyte: Material that provides pure ionic conductivity and physically keep separated fuel and oxidant (dense). Solid Oxide Fuel Cells (SOFCs). Principles The basic reaction in SOFC is: 1 H 2 O2 H 2O G° = -236 kJ/mol (Gibbs’ free energy) (net useful energy available) 2 fuel oxidant exhaust Nernst’s equation: G=-nFE n: number of electrons per mol of product F: Faraday constant (charge of 1 equiv. of electrons) E: cell reaction voltage (OCV: open circuit voltage) (electromotive force of the cell reaction) E = 1.23 V in standard conditions E 1.0 V using air and typical reforming gas (25% H2) If hydrocarbons are used as fuel they must be converted to hydrogen by a reforming reaction. CH4 H 2O CO 3H 2 SOFCs can be directly feeded with hydrocarbons. Reforming of hydrocarbons is promoted at the anodic size of SOFCs using a suitable catalyst due to the high operation temperature. Electrode reactions Anode H 2 O 2 H 2O 2e CO O 2 CO2 2e Cathode 1 O2 2e O 2 2 SOFCs. Polarization phenomena G=-nFE Equilibrium conditions. Only describes the maximum available energy/voltage (OCV) In practice, when the current flows through the circuit, there is a voltage drop due the polarization of the electrodes : = EOCV – ET = 0.3-0.4 V ET = 0.6 – 0.7 V Polarization is determined by irreversibilities (losses) and kinetic limitations. Three effects: Activation polarization: kinetics of electrochemical redox reactions at the electrolyte/electrode interface; Ohmic polarization: resistance of cell components and resistance due to contacts problems; = RI Concentration polarization: arises from limited mass transport capabilities (electrolyte). Typical operating conditions: 0.7 V, 500 mA cm-2 Power = V I = 0.35 W cm-2 Stack of 29 cells, 10x10 cm2: 1kW SOFCs and electrolytes . Two different approaches Oxide-ion conducting electrolyte. Most research and pilot modules are focused on this approach. Proton conducting electrolyte. Lower working temperature but problems of chemical stability and durability still to be solved. SOFCs. Architecture and material requirements Tubular design Planar design Requirements for SOFC materials Very high operation temperatures: 800 (today)-1000°C (1990s). Severe requirements for materials: - Chemically stable in oxidizing and reducing atmospheres; - Absence of interface reaction/diffusion (chemical compatibility); - Similar thermal expansion coefficients; Resistance to thermal cycling - Dimensional stability in the presence of chemical gradients; and stresses SOFCs. Different SOFC architectures Anode supported cell Interconnects supported cell Cathode supported cell Porous substrate (metal foam) supported cell SOFCs. From single cells to stacks Examples of planar SOFC stacks SOFCs. Tubular SOFCs Elements of a micro-tubular SOFC Siemens Westinghouse 100-kW SOFC–CHP power system SOFCs. Materials Present research mainly focused on lowering the working temperature below 800°C to improve reliability, increase life time (target: 40000 h) and reduce costs. Lower temperatures determine: >Slow down of the kinetic processes; >Increase electrode polarization and polarization resistance; LSM: 1 cm2 (1000°C) 1000 cm2 (500°C) >Increase electrolyte resistance; >Reduction of cell voltage, Efficient low-temperature SOFCs require optimization of materials and new combinations of electrolyte and electrode materials for: • Rapid ion transport (thin electrolytes, new electrolytes); • Fast reactions at the electrodes (new cathode materials, optimized microstructure); • Efficient electrocatalysis of oxygen reduction and fuel oxidation Advanced SOFC concept Functional layer: optimized microstructure for long TPB Support layer: coarse porosity and mechanical resistance SOFCs. Materials Kinetic processes at the anode H 2 O 2 H 2O 2e Three-phase percolating composite gas-Ni-YSZ. The hydrogen oxidation reaction occurs at the triple phase boundary (TPB) gas – Ni – YSZ and involves many elementary steps: > Hydrogen adsorption > Surface diffusion > Charge transfert > Water desorption The reaction kinetics is limited by the length of the TPB. TPB length is increased by the use of cermets. Microstructure optimization (small grains, high number of small pores leads to higher performance but increased sensitivity to carbon deposition. 1 CO( g ) C ( s ) O2 ( g ) 2 With pure Ni or noble metal electrodes, hydrogen oxidation only occurs at the metal/YSZ interface rather than in the whole anode volume. SOFCs. Materials Kinetic processes at the cathode 1 O2 2e O 2 2 Kinetic processes: (1) Gas diffusion; (2) O2 adsorption and dissociation; (3) O reduction (4) Solid-state diffusion; (5) Incorporation in the electrolyte at the interface or TPB; Oxygen diffusion coefficient Good electron conductor Poor oxygen conductor Good electron conductor Good oxygen conductor Electrode resistance. Determined by microstructure (tortuosity, porosity, surface area) Surface exchange velocity. Determined by electrode reaction kinetics. SOFCs Overeview of materials and requirements for SOFCs components Component Function Requirements Materials Cathode 1 O2 2e O 2 2 p(O2) = 0.2-1 atm Gas transport Current pick-up Long TPB Porosity Mixed conductivity Catalytic activity for oxygen surface exchange High electrocatalytic activity SrxLa1-xMnO3 (LSM) For T < 800°C: SrxLa1-xCoxFe1-xO3 (LSCF) SrxLa1-xFeO3 (LSF) High density (gas tightness) Pure ionic conductor Mechanical stability Oxide-ion conductors: YxZr1-xO2- (YSZ) GdxCe1-xO2- (GDC) La1-xSrxGa1-yMgyO3 (LSGM) Electrolyte Oxygen ion/proton transport Electronic insulator also mixed with YSZ Compatible with LSM Proton conductors: BaYxCe1-xO3, BaYxZr1-xO3 Anode p(O2) = 10-15-10-20 atm Interconnect H 2 O 2 H 2O 2e Gas transport Current pick-up Electrocatalytic activity for H2 oxidation Current collector Gas distribution Long TPB Porosity Electronic conductivity Redox stability Tolerance to S and C poisoning High electrocatalytic activity Ni-YSZ cermets High electronic conductivity Resistant to oxidation/corrosion Stainless steels Fe-Cr alloys Fe-Al alloys SOFCs. Materials 1 mm Thin electrolyte layer on a anode-supported cell Supporting Ni-YSZ anode with graded porosity Examples of cathodes Electrolyte-supported SOFC SOFCs. Electrolytes 1000K 700K Minimum working temperature for electrolytes (thickness: 10 m; S = 10-2 Scm-1) YSZ: 700 °C; GDC (CGO) and LSGM: 550°C Y:BaZrO3: 400°C; Y:BaCeO3: 550°C Oxide-ion conductors YSZ: YxZr1-xO2- Good oxygen conductivity; High stability and good mechanical properties; Compatible with Ni/NiO electrodes; Reactivity with La-containing perovskites (formation of resistive La2Zr2O7); GDC: GdxCe1-xO2- Highest conductivity at low temperature; Good chemical compatibility with new cobalt-containing cathodes (La0.6Sr0.4Co0.2Fe0.8O3). Electronic conductivity in reducing atmosphere for T > 500°C. LSGM: La1-xSrxGa1-yMgyO3 Higher oxygen conductivity than YSZ Better compatibility with La-containing perovskites; Reactivity with Ni/NiO electrodes. Instability in moist H2. Proton conductors Y:BaZrO3 High bulk conductivity, resistive grain boundaries; Y:BaCeO3 Good conductivity, thermodynamic instability in the presence of CO2 SOFCs. Electrolytes Oxide-ion conductors Ordering phase transitions Use of some electrolytes with high conductivity is limited by phase transitions. The conductive phase is the high-temperature disordered modification. The high temperature phase can be stabilized by appropriate dopants but problems related to instability in reducing conditions and reactivity with electrodes remain. 1670K 1000K 625K Pure electrolytes with order-disorder transition 1670K 1000K Doped electrolytes 625K SOFCs. Electrolytes Oxygen diffusion in perovskites (LaBO3, B=Fe, Cr, Ni, Mn ) A B B Saddle point configuration SOFCs. Electrolytes Oxide-ion conductors YxZr1-xO2- Zr O Cubic Tetragonal Fluorite structure Monoclinic Zr3Y4O12 Y2O3 ZrO 2 2YZr' 3OO VO Y2O3 Optimal compositions: YSZ – YxZr1-xO2- x 0.16 (8 mol.% Y2O3) SSZ – ScxZr1-xO2- x 0.2 (8-12 mol% Sc2O3) (highest conductivity, low defect association energy) SOFCs. Electrolytes Oxide-ion conductors Grain boundary oxygen vacancy segregation in YSZ Real vs. simulated lattice Oxygen column occupancy Column intensity ratio Calculated gb potential barrier: 0.5-1.2 V SOFCs. Electrolytes Oxide-ion conductors Grain boundary oxygen vacancy segregation in YSZ EELS analysis Small angle tilt boundary Column intensity Conductivity (S cm-1) x102 SOFCs. Electrolytes Oxide-ion conductors MxZr1-xO2-δ CaxCe1-xO2-δ M2O3 mol. % Conductivity (S cm-1) Conductivity (S cm-1) CaO mol. % YxCe1-xO2-δ Y2O3 mol. % SOFCs. Electrolytes Oxide-ion conductors Interaction between dopant ions and charge compensating defects with cluster formation is determined by coulombic attraction. The biding energy is strongly modified by lattice relaxation and lattice polarization. For binary oxides with fluorite structure: O Divalent dopant Ca Zr V '' Trivalent dopant Electrical conductivity Ca Zr V Y YZr' VO YZr' VO 2YZr' VO BVO exp H m / RT X O '' VOYZr' Zr ' Prevails at high T and low dopant conc. X zi e i ci For a single charge carrier type: Influence of defect associates on Ea of conductivity of fluorite oxides Dilute range (x <0.08): Defect associations takes place al lower T. Ea is constant (2+ dopants) or decreases (3+ dopants) Concentrated range (x > 0.08): Defect association even at high T. Ea increases with x Z: numero di cariche; e: carica dell’elettrone; : mobilità c: concentrazione A exp E A / RT T Case Y VO Zr ' ' Zr B VO exp H m / RT T Activation energy, Ea Free vacancies Ca VO X Hm Hm + HA2/2 Hm + HA1 Hm : enthalpy of migration HA : binding energy In doped ceria: Hm : 0.6 eV H2 : 0.4-0.6 eV H1 : 0.25 eV SOFCs. Electrolytes Oxide-ion conductors Ceria-based electrolytes (GdxCe1-xO2-δ, GdxCe1-xO2-δ x 0.1). Best electrolytes at 500-600°C Electronic conductivity at low p(O2) (< 10-15 atm at 700°C) ' Gd2O3 CeO 2 2GdCe VO 3OO 1 OO CeO 2 VO 2e ' O2 2 Extrinsic vacancies Intrinsic vacancies ion k pO 1/ 4 2 600°C 700°C SOFCs. Electrolytes Formation of protonic defects Proton conductors Y2O3 2 BaO BaCeO 3 2YCe' 2 Ba Ba VO 5OO BaCeO3 H 2O VO OO 2OH OH K V O p OO OH VO 3 2 O OH O O 2 VO OH M B' 0 H 2O 3K pH 2O K pH 2O 9 K pH 2O K pH 2O S K pH 2O S 2 24S 4S 2 K p H 2O 4 S: effective acceptor concentration = YCe' = water solubility limit Proton conductors Normalized hydration isobars SOFCs. Electrolytes Proton conductors Mobility of protonic defects Two-step transport process: (1) Rotational diffusion of the proton (2) Transfer of the proton to an neighbouring oxide ion by transient formation of an hydrogen bond Transient state Migration activation hentalpies: 0.4 – 0.6 eV Proton mobility strongly sensitive to: • O-O distance; • B-O bond; • Crystallographic distortions; • Acceptor dopant SOFCs. Electrolytes Proton conductors Effect of grain boundaries on ionic conductivity Comparison of ceramics and epitaxial thin films Bulk conductivities of best oxideion and proton conductors wet 5%H2 BaZr0.8Y0.2O3-δ (BZY) 550°C 450°C wet 5%H2 350°C SOFCs. Electrolytes Proton conductors Effect of grain boundaries on ionic conductivity Epitaxial polycrystalline BZY thin films on different substrates MgO substrate. Film orientation: (100) Al2O3 substrate. Film orientation: (111)