Quantum Grand Canonical Ensemble
... which is analogous to F = −kT ln χ. Note that dJ = T dS − p dV + µ dN − d(T S) − d(µN ) = −p dV − S dT − N dµ, ...
... which is analogous to F = −kT ln χ. Note that dJ = T dS − p dV + µ dN − d(T S) − d(µN ) = −p dV − S dT − N dµ, ...
F qvB
... time! But Waves can! Waves add in space and show interference. •Laws of Physics are statistical. •Space and time are relative. •The speed of light is absolute. •Particles are wave-like •Waves are particle-like ...
... time! But Waves can! Waves add in space and show interference. •Laws of Physics are statistical. •Space and time are relative. •The speed of light is absolute. •Particles are wave-like •Waves are particle-like ...
Hydrogen Spectrum
... In this experiment you will determine the wavelengths of the light emitted by a hydrogen lamp. For this task you will use the spectrometer shown in fig.4. This instrument uses a diffraction grating to separate the various wavelengths of light. We will study this optical component in the next experim ...
... In this experiment you will determine the wavelengths of the light emitted by a hydrogen lamp. For this task you will use the spectrometer shown in fig.4. This instrument uses a diffraction grating to separate the various wavelengths of light. We will study this optical component in the next experim ...
The Transactional Interpretation
... small particles such as atoms, electrons, photons, and other subatomic particles. • QM works very well but what it actually tells us about reality is very unclear • An interpretation is intended to make clear what the theory tells us about reality ...
... small particles such as atoms, electrons, photons, and other subatomic particles. • QM works very well but what it actually tells us about reality is very unclear • An interpretation is intended to make clear what the theory tells us about reality ...
Nonlinear wave mechanics of complex material systems
... E being evaluated for the same quantity of matter, say an elementary reference volume of a continuum. In 1923 L. de Broglie recognized (see [1]) that the action S is a space-time invariant. Considering the Planck–Einstein relation E = hω , where h = h / 2π is the Planck reduced constant, the quantum ...
... E being evaluated for the same quantity of matter, say an elementary reference volume of a continuum. In 1923 L. de Broglie recognized (see [1]) that the action S is a space-time invariant. Considering the Planck–Einstein relation E = hω , where h = h / 2π is the Planck reduced constant, the quantum ...
PART 1 Identical particles, fermions and bosons. Pauli exclusion
... * Particles with integer spin have R = 1, they are called bosons (Bose - Einstein statistics). * Particles with half-integer spins have R = −1, they are called fermions (Fermi - Dirac statistics). ...
... * Particles with integer spin have R = 1, they are called bosons (Bose - Einstein statistics). * Particles with half-integer spins have R = −1, they are called fermions (Fermi - Dirac statistics). ...
Solution key to exam 1 - University of Rochester
... has a mass of 2 kg and carries a positive electrical charge of +1 Coulombs. Particle B has a mass of 4 kg and carries a charge -1.5 Coulombs. a) On the sketch below, indicate with a little arrow the direction of the electric field due to particle A at the point where particle B is located. b) On the ...
... has a mass of 2 kg and carries a positive electrical charge of +1 Coulombs. Particle B has a mass of 4 kg and carries a charge -1.5 Coulombs. a) On the sketch below, indicate with a little arrow the direction of the electric field due to particle A at the point where particle B is located. b) On the ...
Document
... It should not be surprising to find that the penetration distance that violates classical physics is proportional to Planck’s constant. ...
... It should not be surprising to find that the penetration distance that violates classical physics is proportional to Planck’s constant. ...
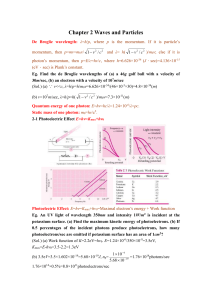
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
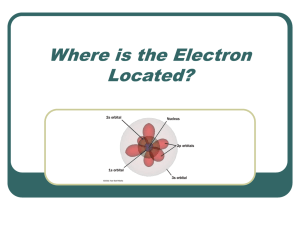
III. Quantum Model of the Atom
... Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: ...
... Pauli Exclusion Principle No two electrons in an atom can have the same 4 quantum numbers. Each e- has a unique “address”: ...
What is a photon, really - Philsci-Archive
... Department of Physics and Astronomy, University of Pittsburgh Our early training in physics encourages us to imagine photons as little pellets flying through the air, and to see wave-particle duality as a paradox. This view persists from the debates on quantum mechanics early in the 20th century. Mu ...
... Department of Physics and Astronomy, University of Pittsburgh Our early training in physics encourages us to imagine photons as little pellets flying through the air, and to see wave-particle duality as a paradox. This view persists from the debates on quantum mechanics early in the 20th century. Mu ...
Electronic structure (download)
... the atom lay in accepting the wave properties of electrons De Broglie wave-particle duality All particles have a wavelength – wavelike nature. – Significant only for very small particles – like electrons or photons – As mass increases, wavelength decreases ...
... the atom lay in accepting the wave properties of electrons De Broglie wave-particle duality All particles have a wavelength – wavelike nature. – Significant only for very small particles – like electrons or photons – As mass increases, wavelength decreases ...
INTRODUCTION TO QUANTUM MECHANICS I I mention in class
... I mention in class how the energy eigenvalues in one dimension are never degenerate. That may seem counterintuitive. In fact, consider two identical potential wells separated by a vast distance. One could imagine that a wave function concentrated around one well would be degenerate in energy with an ...
... I mention in class how the energy eigenvalues in one dimension are never degenerate. That may seem counterintuitive. In fact, consider two identical potential wells separated by a vast distance. One could imagine that a wave function concentrated around one well would be degenerate in energy with an ...
Challenging Modern Physics
... “Bohr is in the habit of saying: the wave and corpuscular views are complementary. By this he means: if we prove the corpuscular character of an experiment, then it is impossible at the same time to prove its wave character, and conversely.” ...
... “Bohr is in the habit of saying: the wave and corpuscular views are complementary. By this he means: if we prove the corpuscular character of an experiment, then it is impossible at the same time to prove its wave character, and conversely.” ...
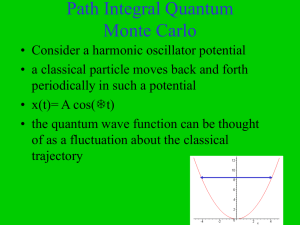
Path Integral Quantum Monte Carlo
... periodically in such a potential • x(t)= A cos(t) • the quantum wave function can be thought of as a fluctuation about the classical trajectory ...
... periodically in such a potential • x(t)= A cos(t) • the quantum wave function can be thought of as a fluctuation about the classical trajectory ...