Electromagnetic Packet
... In the early 1920’s it was becoming apparent that there were some difficulties with the Bohr model of the atom. One difficulty was that Bohr used classical physics to calculate the orbits of the hydrogen atom but this could not be used to explain the ability of electrons to stay in only certain ener ...
... In the early 1920’s it was becoming apparent that there were some difficulties with the Bohr model of the atom. One difficulty was that Bohr used classical physics to calculate the orbits of the hydrogen atom but this could not be used to explain the ability of electrons to stay in only certain ener ...
ppt
... in any given energy level and arrows for electrons Unoccupied circles imply that there are no electrons in it A circle can have at most two electrons in it; only if the arrows are pointing in opposite ...
... in any given energy level and arrows for electrons Unoccupied circles imply that there are no electrons in it A circle can have at most two electrons in it; only if the arrows are pointing in opposite ...
Optical Control and Info
... excitations in semiconductors (excitons) in confined and/or periodic nanostructures, such as quantum wells, quantum wires, or arrays of quantum dots. Cavity polaritons are states resulting from the strong coupling of excitons with photons in an electromagnetic cavity. Both excitons and cavity polari ...
... excitations in semiconductors (excitons) in confined and/or periodic nanostructures, such as quantum wells, quantum wires, or arrays of quantum dots. Cavity polaritons are states resulting from the strong coupling of excitons with photons in an electromagnetic cavity. Both excitons and cavity polari ...
Remarks on Energy in the Many Worlds
... Thus the experimentally relevant measure of multiverse energy is not the simple sum of the two universe energies, but rather a weighted average. It is perfectly consistent with Eqn. (9), for example, to have E = E1 = E2 . So the answer is that the “extra energy” doesn’t have to come from anywhere, b ...
... Thus the experimentally relevant measure of multiverse energy is not the simple sum of the two universe energies, but rather a weighted average. It is perfectly consistent with Eqn. (9), for example, to have E = E1 = E2 . So the answer is that the “extra energy” doesn’t have to come from anywhere, b ...
Assignment 8 - Duke Physics
... electron is found inside a small cube of volume dV1 centered on the vector x1 , the second electron is found inside a small cube of volume dV2 centered on the vector x2 , the first proton is found inside a small cube of volume dV3 centered on the vector x3 , and the second proton is found inside a s ...
... electron is found inside a small cube of volume dV1 centered on the vector x1 , the second electron is found inside a small cube of volume dV2 centered on the vector x2 , the first proton is found inside a small cube of volume dV3 centered on the vector x3 , and the second proton is found inside a s ...
quantum physics - Enggphysicsvenkat
... in air, pressure varies in space and time P(x, t). For EM waves, E and B are varying in space and time. To characterise de Broglie waves associated with a material particle, which require a quantity that varies in space and time. That quantity is called as “Wave Function”, designated by “ψ”, which i ...
... in air, pressure varies in space and time P(x, t). For EM waves, E and B are varying in space and time. To characterise de Broglie waves associated with a material particle, which require a quantity that varies in space and time. That quantity is called as “Wave Function”, designated by “ψ”, which i ...
Quantum Chemistry - Winona State University
... Postulates of Quantum Theory • The state of a system is defined by a function (usually denoted and called the wavefunction or state function) that contains all the information that can be known about the system. • Every physical observable is represented by a linear operator called the “Hermitian ...
... Postulates of Quantum Theory • The state of a system is defined by a function (usually denoted and called the wavefunction or state function) that contains all the information that can be known about the system. • Every physical observable is represented by a linear operator called the “Hermitian ...
Lab Science 9 Pacing Guide
... 12. Explain how an object's kinetic energy depends on its mass and its speed (KE=½mv 2). 13. Demonstrate that near Earth's surface an object's gravitational potential energy depends upon its weight (mg where m is the object's mass and g is the acceleration due to gravity) and height (h) above a refe ...
... 12. Explain how an object's kinetic energy depends on its mass and its speed (KE=½mv 2). 13. Demonstrate that near Earth's surface an object's gravitational potential energy depends upon its weight (mg where m is the object's mass and g is the acceleration due to gravity) and height (h) above a refe ...
1993 AP Physics B Free-Response
... c. The change in internal energy Ub - Ua d. The work Wbc done by the gas on its surroundings during process bc The net heat added to the gas for the entire cycle 1,800 joules. Determine each of the following. e. The net work done by the gas on its surroundings for the entire cycle f. The efficiency ...
... c. The change in internal energy Ub - Ua d. The work Wbc done by the gas on its surroundings during process bc The net heat added to the gas for the entire cycle 1,800 joules. Determine each of the following. e. The net work done by the gas on its surroundings for the entire cycle f. The efficiency ...
OCET-2012 Question Booklet Series : A Roll No. Subject :
... 6. Each question has four alternative answers (A, B, C, D) of which only one is correct. For each question, darken only one bubble (A or B or C or D), whichever you think is the correct answer, on the Answer Sheet with Black Ball Point / Black Gel pen. 7. If you do not want to answer a question, lea ...
... 6. Each question has four alternative answers (A, B, C, D) of which only one is correct. For each question, darken only one bubble (A or B or C or D), whichever you think is the correct answer, on the Answer Sheet with Black Ball Point / Black Gel pen. 7. If you do not want to answer a question, lea ...
General Chemistry: An Integrated Approach
... • #1. Electrons can be bent, or diffracted, like light waves. • #2. Electrons bumping into each other can cause interference, when beams of electrons overlap. • Interference can cause either a reduction in energy or an increase in energy. ...
... • #1. Electrons can be bent, or diffracted, like light waves. • #2. Electrons bumping into each other can cause interference, when beams of electrons overlap. • Interference can cause either a reduction in energy or an increase in energy. ...
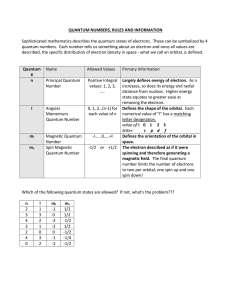
Quantum Number Table
... increases, so does its energy and radial distance from nucleus. Higher energy state equates to greater ease at removing the electron. Defines the shape of the orbital. Each numerical value of "l" has a matching letter designation. value of l: 0 1 2 3 letter: s p d f Defines the orientation of the or ...
... increases, so does its energy and radial distance from nucleus. Higher energy state equates to greater ease at removing the electron. Defines the shape of the orbital. Each numerical value of "l" has a matching letter designation. value of l: 0 1 2 3 letter: s p d f Defines the orientation of the or ...
Waves QM and SCh eq
... These waves are governed by Newton’s laws, and they can exist only within a material medium, such as air, water, and rock. 2. Electromagnetic waves. Examples include visible and ultraviolet light, radio and television waves, microwaves, x rays, and radar waves. These waves require no material medium ...
... These waves are governed by Newton’s laws, and they can exist only within a material medium, such as air, water, and rock. 2. Electromagnetic waves. Examples include visible and ultraviolet light, radio and television waves, microwaves, x rays, and radar waves. These waves require no material medium ...
Document
... (1) A particle moves forward in time, emits two photons at ( x2 , t2 ) and moves back in time with negative energy to point ( x1 , t1 ) where it scatters off a photon and moves forward in time. There is only one particle moving through space and time. (2) At point ( x1 , t1 ) an antiparticle-particl ...
... (1) A particle moves forward in time, emits two photons at ( x2 , t2 ) and moves back in time with negative energy to point ( x1 , t1 ) where it scatters off a photon and moves forward in time. There is only one particle moving through space and time. (2) At point ( x1 , t1 ) an antiparticle-particl ...
44. Quantum Energy Wave Function Equation
... KEYWORDS: Quantum energy, wave function; Harmonic oscillator. I. ...
... KEYWORDS: Quantum energy, wave function; Harmonic oscillator. I. ...
2·QUIZLET VOCABULARY: Quantum Numbers Study online at
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
Practice Final Spring 2016
... 2. The object in the sky that lies very nearly on an extension of the earth's axis is A. the sun. B. Orion. C. Mercury. D. Polaris 3. In which one or more of the following is the earth assumed to be the center of the universe? A. the Ptolemaic system B. the Copernican system C. Kepler's laws of plan ...
... 2. The object in the sky that lies very nearly on an extension of the earth's axis is A. the sun. B. Orion. C. Mercury. D. Polaris 3. In which one or more of the following is the earth assumed to be the center of the universe? A. the Ptolemaic system B. the Copernican system C. Kepler's laws of plan ...
Applied quantum mechanics 1 Applied Quantum Mechanics
... (d) Show that E kinetic = – E potential 2 (which is a result predicted by the virial theorem). (e) Show that the peak in radial probability occurs at r = a B Z . (f) Show that the expectation value r = 3a B 2Z . (g) Show that the expectation value of momentum p = 0 . Problem 11.3 T ...
... (d) Show that E kinetic = – E potential 2 (which is a result predicted by the virial theorem). (e) Show that the peak in radial probability occurs at r = a B Z . (f) Show that the expectation value r = 3a B 2Z . (g) Show that the expectation value of momentum p = 0 . Problem 11.3 T ...
Answers
... 3) A beam of tennis balls are being fired through two slits toward a wall. Sketch what it will look like if the balls leave a mark on the wall. Which pattern above can represent the density of marks? What you see will be a bunch of dots. They will show either of the first two patterns, depending on ...
... 3) A beam of tennis balls are being fired through two slits toward a wall. Sketch what it will look like if the balls leave a mark on the wall. Which pattern above can represent the density of marks? What you see will be a bunch of dots. They will show either of the first two patterns, depending on ...
Atomic and Molecular Spectroscopy
... o The Physics of Atoms and Quanta: Introduction to experiement and theory Haken & Wolf (Springer) o Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles Eisberg & Resnick (Wiley) o Quantum Mechanics McMurry QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture ...
... o The Physics of Atoms and Quanta: Introduction to experiement and theory Haken & Wolf (Springer) o Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles Eisberg & Resnick (Wiley) o Quantum Mechanics McMurry QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture ...