Quantum mechanics and electron structure
... Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstract mathematical model remains a fascinating, complex and unresolved argument ...
... Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstract mathematical model remains a fascinating, complex and unresolved argument ...
Lecture-XXIII Quantum Mechanics
... Classically the state of a particle is defined by (x,p) and the dynamics is given by Hamilton’s equations ...
... Classically the state of a particle is defined by (x,p) and the dynamics is given by Hamilton’s equations ...
Probing contextuality with superconducting quantum circuits Talk 27. Oct. 2015 ABSTRACT:
... Contextuality is one of the most fundamental property which distinguishes quantum mechanics from classical theory. It has also been suggested to be the 'magical' resource responsible for an exponential speedup of a quantum computer. We will provide the first experimental evidence of this resource fo ...
... Contextuality is one of the most fundamental property which distinguishes quantum mechanics from classical theory. It has also been suggested to be the 'magical' resource responsible for an exponential speedup of a quantum computer. We will provide the first experimental evidence of this resource fo ...
What`s the Matter?: Quantum Physics for Ordinary People
... Light: Particle or Wave? Albert Einstein explained the photoelectric effect by showing that light energy is quantized. Light, even while exhibiting wave-like interference, comes in particle-like energy packets called photons. What are photons? Certainly not classical particles. When traveling throu ...
... Light: Particle or Wave? Albert Einstein explained the photoelectric effect by showing that light energy is quantized. Light, even while exhibiting wave-like interference, comes in particle-like energy packets called photons. What are photons? Certainly not classical particles. When traveling throu ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
Bohr Model and Principal Quantum Number
... The units of Planck’s constant can be broken down into kg, m and m/s which would be the product of mass, distance and ...
... The units of Planck’s constant can be broken down into kg, m and m/s which would be the product of mass, distance and ...
Quantum Mechanics Physics
... • Einstein also proposed that electrons, besides emitting electromagnetic radiation in quanta, also absorb it in quanta. • Einstein's work demonstrated that electromagnetic radiation has the characteristics of both a wave--because the fields of which it is composed rise and fall in strength--and a p ...
... • Einstein also proposed that electrons, besides emitting electromagnetic radiation in quanta, also absorb it in quanta. • Einstein's work demonstrated that electromagnetic radiation has the characteristics of both a wave--because the fields of which it is composed rise and fall in strength--and a p ...
The Fall 2005 Qualifying Exam, Part 1
... Work 3 out of the 5 problems, problem 11 – problem 15. Problem 11: You are given a microphone hooked up to a frequency analyzer and asked to measure the sound velocity in a steel bar of length L = 0.5m by measuring the frequency of sound waves generated when the bar is struck by a steel mallet. (a) ...
... Work 3 out of the 5 problems, problem 11 – problem 15. Problem 11: You are given a microphone hooked up to a frequency analyzer and asked to measure the sound velocity in a steel bar of length L = 0.5m by measuring the frequency of sound waves generated when the bar is struck by a steel mallet. (a) ...
Problem set 7
... 5. Consider a particle in a (real) potential V(x). Suppose ψ(x) is a solution of the time-independent Schrödinger equation with (real) energy eigenvalue E . Find another wave function that has the same eigenvalue E . When are the two eigenfunctions the same? 6. Use the result of the previous proble ...
... 5. Consider a particle in a (real) potential V(x). Suppose ψ(x) is a solution of the time-independent Schrödinger equation with (real) energy eigenvalue E . Find another wave function that has the same eigenvalue E . When are the two eigenfunctions the same? 6. Use the result of the previous proble ...
Modern Physics: Quantum Mechanics
... • Spectrum has shifted so that colors are more equally represented — white hot ...
... • Spectrum has shifted so that colors are more equally represented — white hot ...
Quantum Mechanics
... characteristics of waves. – He postulated that a particle with mass m and a velocity v has an associated wavelength. – The equation λ = h/mv is called the de Broglie relation. ...
... characteristics of waves. – He postulated that a particle with mass m and a velocity v has an associated wavelength. – The equation λ = h/mv is called the de Broglie relation. ...
Chapter 3 notes
... Thompson discovered the electron and proposed a model for the structure of the atom. Using a CATHODE RAY TUBE, ...
... Thompson discovered the electron and proposed a model for the structure of the atom. Using a CATHODE RAY TUBE, ...
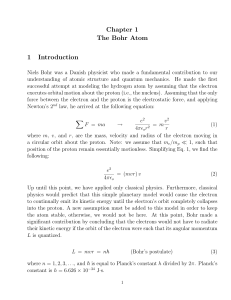
Chapter 1 The Bohr Atom 1 Introduction
... physics would predict that this simple planetary model would cause the electron to continually emit its kinetic energy until the electron’s orbit completely collapses into the proton. A new assumption must be added to this model in order to keep the atom stable, otherwise, we would not be here. At t ...
... physics would predict that this simple planetary model would cause the electron to continually emit its kinetic energy until the electron’s orbit completely collapses into the proton. A new assumption must be added to this model in order to keep the atom stable, otherwise, we would not be here. At t ...
do with electron orbitals?
... II. The angular momentum of the ground state solution is different _______ different III. The location of the electron is _______ a. same, same, same b. same, same, different c. same, different, different d. different, same, different e. different, different, different ...
... II. The angular momentum of the ground state solution is different _______ different III. The location of the electron is _______ a. same, same, same b. same, same, different c. same, different, different d. different, same, different e. different, different, different ...
CHAPTER 5 NOTES – ELECTRONS IN ATOMS
... • c = λν • The wavelength and frequency of light are inversely proportional to each other • c = speed of light (3E8 m/s or 3 E10 cm/s) • When atoms absorb energy, electrons move into higher energy levels, and these electrons lose energy by emitting light when they return to lower energy levels. ...
... • c = λν • The wavelength and frequency of light are inversely proportional to each other • c = speed of light (3E8 m/s or 3 E10 cm/s) • When atoms absorb energy, electrons move into higher energy levels, and these electrons lose energy by emitting light when they return to lower energy levels. ...
Physics of wave packets
... formula ), Rydberg atoms are expected to exist. They have large van der Waals force and large photon absorption rate. ...
... formula ), Rydberg atoms are expected to exist. They have large van der Waals force and large photon absorption rate. ...