Chapter 6 Outline full
... • It cannot explain the spectra of atoms other than hydrogen. • Electrons do not move about the nucleus in circular orbits. ...
... • It cannot explain the spectra of atoms other than hydrogen. • Electrons do not move about the nucleus in circular orbits. ...
Conduction and Semiconductors
... Where h is Planck’s constant (h = 6.626 x 10-34 J-s). What this means is that if an object is giving off (or absorbing) light it is actually emitting (absorbing) photons. The energy of each photon is dependent only upon its frequency (or wavelength or color). In 1925, Louis DeBroglie hypothesized th ...
... Where h is Planck’s constant (h = 6.626 x 10-34 J-s). What this means is that if an object is giving off (or absorbing) light it is actually emitting (absorbing) photons. The energy of each photon is dependent only upon its frequency (or wavelength or color). In 1925, Louis DeBroglie hypothesized th ...
Quantum physics
... breaks down and the relativistic treatment discovered by Einstein must be used. Now, we will see that the description of light entirely in terms of waves breaks down at very small scales. In addition, we will see that objects that have mass, which we usually think of as particles (like electrons ...
... breaks down and the relativistic treatment discovered by Einstein must be used. Now, we will see that the description of light entirely in terms of waves breaks down at very small scales. In addition, we will see that objects that have mass, which we usually think of as particles (like electrons ...
Chapter 6: Electronic Structure of Atoms Recommended Text
... electronic configuration of 10B differ from that of 11B? Draw the orbital diagram for an atom of 11B. Which electrons are the valence electrons? Indicate three major ways in which the 1s electrons in boron differ from its 2s electrons. Elemental boron reacts with fluorine to form BF3, a gas. Write a ...
... electronic configuration of 10B differ from that of 11B? Draw the orbital diagram for an atom of 11B. Which electrons are the valence electrons? Indicate three major ways in which the 1s electrons in boron differ from its 2s electrons. Elemental boron reacts with fluorine to form BF3, a gas. Write a ...
PPT
... N independent trials are made of a quantity x. The possible results form a discrete spectrum x1 , x2 , ... xi , ... xM (M possible distinct results). Out of N trials, ni of the trials produce result xi. If you add up all the results of all N trials, what is the sum of the results? ...
... N independent trials are made of a quantity x. The possible results form a discrete spectrum x1 , x2 , ... xi , ... xM (M possible distinct results). Out of N trials, ni of the trials produce result xi. If you add up all the results of all N trials, what is the sum of the results? ...
CHAPTER 3: The Experimental Basis of Quantum Theory
... depends on the value of the light frequency f and not on the intensity. The existence of a threshold frequency is completely inexplicable in classical theory. Classical theory would predict that for extremely low light intensities, a long time would elapse before any one electron could obtain suffic ...
... depends on the value of the light frequency f and not on the intensity. The existence of a threshold frequency is completely inexplicable in classical theory. Classical theory would predict that for extremely low light intensities, a long time would elapse before any one electron could obtain suffic ...
Chemistry 871/671/495, Structure and Bonding
... world. To understand the structure of molecules and their reactivity, one has no choice but to rely on quantum mechanics. In this course, we will introduce quantum mechanical principles and their applications to atomic and molecular systems. We will start by discussing the failures of classical phys ...
... world. To understand the structure of molecules and their reactivity, one has no choice but to rely on quantum mechanics. In this course, we will introduce quantum mechanical principles and their applications to atomic and molecular systems. We will start by discussing the failures of classical phys ...
Class25_review - Rensselaer Polytechnic Institute
... Derived in 1917 by Einstein. (Required for thermal equilibrium was it was recognized that photons were quantized.) However, a “real” understanding of this was not achieved until the 1950’s. ...
... Derived in 1917 by Einstein. (Required for thermal equilibrium was it was recognized that photons were quantized.) However, a “real” understanding of this was not achieved until the 1950’s. ...
Lecture
... Spin quantum number “s” is a unique property of a particle. Fermions have half integer value of “s”. Two fermions cannot occupy the same quantum state. Electron, Proton, Neutron: s=1/2 Bosons have full integer value of “s”. There is no limitation in the number of bosons that can occupy the same stat ...
... Spin quantum number “s” is a unique property of a particle. Fermions have half integer value of “s”. Two fermions cannot occupy the same quantum state. Electron, Proton, Neutron: s=1/2 Bosons have full integer value of “s”. There is no limitation in the number of bosons that can occupy the same stat ...
Chapter 5
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the waveparticle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the or ...
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the waveparticle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the or ...
Chapter 6:Electronic Structure of Atoms
... photon must have an energy equal to Planck’s constant times the frequency of the light. • Analysis of data from the photoelectric experiment showed that the energy of the ejected electrons was proportional to the frequency of the illuminating light. This showed that whatever was knocking the electro ...
... photon must have an energy equal to Planck’s constant times the frequency of the light. • Analysis of data from the photoelectric experiment showed that the energy of the ejected electrons was proportional to the frequency of the illuminating light. This showed that whatever was knocking the electro ...
Electron Orbital
... The Electrons: wave or particle Electrons display properties of both. To think of them as a particle is easy because they have a small amount of mass. There is evidence of wave behavior though. In this sense they are neither particles nor waves in the absolute sense, but only exhibit wave or ...
... The Electrons: wave or particle Electrons display properties of both. To think of them as a particle is easy because they have a small amount of mass. There is evidence of wave behavior though. In this sense they are neither particles nor waves in the absolute sense, but only exhibit wave or ...
The photoelectric effect - University of Toronto Physics
... - if the potential difference ΔV is made negative (anode negative with respect to the cathode), current I decreases until it becomes zero (at ΔV= -Vstop). Vstop is called stopping potential. The value of Vstop is the same for intense and weak light. Classical interpretation Classical physics tried t ...
... - if the potential difference ΔV is made negative (anode negative with respect to the cathode), current I decreases until it becomes zero (at ΔV= -Vstop). Vstop is called stopping potential. The value of Vstop is the same for intense and weak light. Classical interpretation Classical physics tried t ...
wave
... quantum system stop existing as a mixture of states and become one or the other? (More technically, when does the actual quantum state stop being a linear combination of states, each of which resemble different classical states, and instead begin to have a unique classical description?) If the cat s ...
... quantum system stop existing as a mixture of states and become one or the other? (More technically, when does the actual quantum state stop being a linear combination of states, each of which resemble different classical states, and instead begin to have a unique classical description?) If the cat s ...
Introduction to Quantum Mechanic
... In physics and chemistry, wave–particle duality is the concept that all matter and energy exhibits both wave-like and particle-like properties. A central concept of quantum mechanics, duality, addresses the inadequacy of classical concepts like "particle" and "wave" in fully describing the behavior ...
... In physics and chemistry, wave–particle duality is the concept that all matter and energy exhibits both wave-like and particle-like properties. A central concept of quantum mechanics, duality, addresses the inadequacy of classical concepts like "particle" and "wave" in fully describing the behavior ...
Chemistry 1000 Lecture 6: Quantum mechanics and spectroscopy
... Chemistry 1000 Lecture 6: Quantum mechanics and spectroscopy Marc R. Roussel ...
... Chemistry 1000 Lecture 6: Quantum mechanics and spectroscopy Marc R. Roussel ...
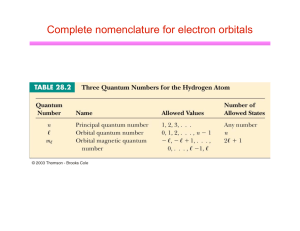
Complete nomenclature for electron orbitals
... that angular momentum of the hydrogen atom is quantized: mevr = nh • Why? Not known for 10 years until de Broglie gave a physical interpretation. • Electron orbit could be stable only if an integral number of electron wavelengths could be fit inside orbit. • 2pr = nl n=1,2,3,… ...
... that angular momentum of the hydrogen atom is quantized: mevr = nh • Why? Not known for 10 years until de Broglie gave a physical interpretation. • Electron orbit could be stable only if an integral number of electron wavelengths could be fit inside orbit. • 2pr = nl n=1,2,3,… ...