Budiansky Cover
... "Once at the end of a colloquium I heard Debye saying something like: Schrödinger, you are not working right now on very important problems anyway. Why don't you tell us sometime about that thesis of de Broglie? "So in one of the next colloquia, Schrödinger gave a beautifully clear account of how de ...
... "Once at the end of a colloquium I heard Debye saying something like: Schrödinger, you are not working right now on very important problems anyway. Why don't you tell us sometime about that thesis of de Broglie? "So in one of the next colloquia, Schrödinger gave a beautifully clear account of how de ...
Cavendish Laboratory
... • Study models and potentially applicable systems systems made by both chemistry (e.g. cuprates, manganites, heavy fermions) and physics (e.g. quantum wells, optical lattices) extreme conditions useful (B,T,P) ...
... • Study models and potentially applicable systems systems made by both chemistry (e.g. cuprates, manganites, heavy fermions) and physics (e.g. quantum wells, optical lattices) extreme conditions useful (B,T,P) ...
Sample pages 1 PDF
... wavelength ranges of the spectrum. In his analysis Planck assumed that the energy changed by quanta proportional to a constant denoted as h, which was later named the Planck constant. The dependence proposed by Planck described the measured emission of electromagnetic radiation much better than the ...
... wavelength ranges of the spectrum. In his analysis Planck assumed that the energy changed by quanta proportional to a constant denoted as h, which was later named the Planck constant. The dependence proposed by Planck described the measured emission of electromagnetic radiation much better than the ...
Lecture 27: Quantum Mechanics (Continued)
... Then the question becomes what wavelengths are acceptable? If we assume that the nodes of the wave must be present at the box boundaries then it immediately implies not all wavelengths can be accepted, i.e. wavelength is quantized just as in the case of a standing wave problem. The acceptable wavele ...
... Then the question becomes what wavelengths are acceptable? If we assume that the nodes of the wave must be present at the box boundaries then it immediately implies not all wavelengths can be accepted, i.e. wavelength is quantized just as in the case of a standing wave problem. The acceptable wavele ...
Potential Step: Griffiths Problem 2.33 Prelude: Note that the time
... This second-order differential equation is difficult to solve even for simple potentials encountered in classical mechanics, e.g., a charged particle in a constant electric field, V (x) = −qEx which leads to a constant force (i.e., constant acceleration, x = x0 + v0 t + (1/2)at2 and all that!) or th ...
... This second-order differential equation is difficult to solve even for simple potentials encountered in classical mechanics, e.g., a charged particle in a constant electric field, V (x) = −qEx which leads to a constant force (i.e., constant acceleration, x = x0 + v0 t + (1/2)at2 and all that!) or th ...
Quantum Mechanics - University of Colorado Boulder
... doing?" or "Where is the particle?" Instead, we can only ask about the possible results of measurements: "If I make a measurement, what is the probability that I will get such-andsuch a result?" QM is all about measurement, which is the only way we ever truly know anything about the physical univers ...
... doing?" or "Where is the particle?" Instead, we can only ask about the possible results of measurements: "If I make a measurement, what is the probability that I will get such-andsuch a result?" QM is all about measurement, which is the only way we ever truly know anything about the physical univers ...
Basics of wave functions - Department of Physics | Oregon State
... Quantum Mechanics – kets and operators The state of electron is represented by a quantity called a state vector or a ket, y , which in general is a function of many variables, including time. In PH425, you learned about kets that contained information about a particle’s spin state. We’ll be interest ...
... Quantum Mechanics – kets and operators The state of electron is represented by a quantity called a state vector or a ket, y , which in general is a function of many variables, including time. In PH425, you learned about kets that contained information about a particle’s spin state. We’ll be interest ...
B.3 Time dependent quantum mechanics
... oscillator eigenstates. They will tend to add up on the left, and cancel on the right (because odd/even quantum number eigenstates switch ‘lobes’ from positive to negative on the right side). The resulting wave packet is localized on the left side. As it time-evolves, the phases of the higher energy ...
... oscillator eigenstates. They will tend to add up on the left, and cancel on the right (because odd/even quantum number eigenstates switch ‘lobes’ from positive to negative on the right side). The resulting wave packet is localized on the left side. As it time-evolves, the phases of the higher energy ...
Chapter 7: Quantum Mechanical Model of Atom
... • Werner Heisenberg - showed that it is impossible to know (or measure) precisely both the position and velocity (or the momentum) at the same time. • The simple act of “seeing” an electron would change ...
... • Werner Heisenberg - showed that it is impossible to know (or measure) precisely both the position and velocity (or the momentum) at the same time. • The simple act of “seeing” an electron would change ...
2/25/11 QUANTUM MECHANICS II (524) PROBLEM SET 6 (hand in
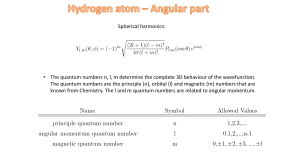
... electron). The electron angular momentum is denoted by J = L + S, where L is the orbital angular momentum of the electron and S its spin. The total angular momentum of the atom is F = J + I, where I is the nuclear spin. a) What are the possible values of the quantum numbers J and F for a deuterium a ...
... electron). The electron angular momentum is denoted by J = L + S, where L is the orbital angular momentum of the electron and S its spin. The total angular momentum of the atom is F = J + I, where I is the nuclear spin. a) What are the possible values of the quantum numbers J and F for a deuterium a ...
quantum mechanics and real events - Heriot
... is deterministic, continuous, and symmetric under time reversal. But when an observation or measurement is made, the state vector changes according to a different rule which is probabilistic, discontinuous, and asymmetric under time reversal. As long as we stay in the physics laboratory, we know wha ...
... is deterministic, continuous, and symmetric under time reversal. But when an observation or measurement is made, the state vector changes according to a different rule which is probabilistic, discontinuous, and asymmetric under time reversal. As long as we stay in the physics laboratory, we know wha ...
Do we need the Concept of Particle?
... part of the measurement device. One must only note that the wavemechanical model has no ambition other than providing a method to calculate the probabilities which are needed at the end of the process. It does not offer a description of the intermediate events between the preparation of an experimen ...
... part of the measurement device. One must only note that the wavemechanical model has no ambition other than providing a method to calculate the probabilities which are needed at the end of the process. It does not offer a description of the intermediate events between the preparation of an experimen ...
“What is quantum theory about?” Jos Uffink March 26, 2010, Utrecht
... where Sd is the single-particle state space, and P(ρ) some probability density over Sd . ...
... where Sd is the single-particle state space, and P(ρ) some probability density over Sd . ...
BORH`S DERIVATION OF BALMER
... The fact that a purely chance agreement between the quantities in equation (13) is highly improbable, lends plausibility to Bohr’s quantum theory of the hydrogen atom. This theory gave great impetus to quantum mechanics, in its ability to explain the motion of atomic charged particles and was recogn ...
... The fact that a purely chance agreement between the quantities in equation (13) is highly improbable, lends plausibility to Bohr’s quantum theory of the hydrogen atom. This theory gave great impetus to quantum mechanics, in its ability to explain the motion of atomic charged particles and was recogn ...
LESSON 9
... thought to be essentially static, and unchanging in time. On the other hand, it must have been obvious, that society is evolving in culture and technology. This indicates that the present phase of human history can not have been going for more than a few thousand years. Otherwise, we would be more a ...
... thought to be essentially static, and unchanging in time. On the other hand, it must have been obvious, that society is evolving in culture and technology. This indicates that the present phase of human history can not have been going for more than a few thousand years. Otherwise, we would be more a ...