The One-Dimensional Finite-Difference Time
... For a potential barrier of thickness T , the potential function is simply defined as V (x) = V0 , where −T /2 ≤ x ≤ T /2, and V0 is some potential energy that is only slightly greater than KE. Figure 1 shows a simulated demonstration of just such a system. A wave packet with mean kinetic energy of K ...
... For a potential barrier of thickness T , the potential function is simply defined as V (x) = V0 , where −T /2 ≤ x ≤ T /2, and V0 is some potential energy that is only slightly greater than KE. Figure 1 shows a simulated demonstration of just such a system. A wave packet with mean kinetic energy of K ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
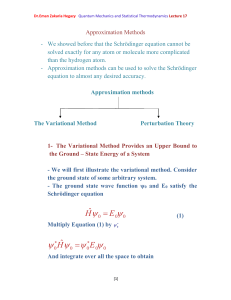
... 1- The Variational Method Provides an Upper Bound to the Ground – State Energy of a System - We will first illustrate the variational method. Consider the ground state of some arbitrary system. - The ground state wave function ψ0 and E0 satisfy the Schrödinger equation ...
... 1- The Variational Method Provides an Upper Bound to the Ground – State Energy of a System - We will first illustrate the variational method. Consider the ground state of some arbitrary system. - The ground state wave function ψ0 and E0 satisfy the Schrödinger equation ...
Recap of Lectures 12-2
... at high quantum number n. Truly classical behaviour (observable change with time) requires physical state to be a superposition of energy states. ...
... at high quantum number n. Truly classical behaviour (observable change with time) requires physical state to be a superposition of energy states. ...
qm1-web - Michael Nielsen
... How successful is quantum mechanics? It is unbelievably successful. Not just for the small stuff! QM crucial to explain why stars shine, how the Universe formed, and the stability of matter. No deviations from quantum mechanics are known Most physicists believe that any “theory of everything” will ...
... How successful is quantum mechanics? It is unbelievably successful. Not just for the small stuff! QM crucial to explain why stars shine, how the Universe formed, and the stability of matter. No deviations from quantum mechanics are known Most physicists believe that any “theory of everything” will ...
Quantum Mechanics I: Basic Principles
... Basic Principles Michael A. Nielsen University of Queensland “I ain’t no physicist but I know what matters” - Popeye the Sailor ...
... Basic Principles Michael A. Nielsen University of Queensland “I ain’t no physicist but I know what matters” - Popeye the Sailor ...
PARTICLE IN AN INFINITE POTENTIAL WELL
... This process may be performed for any other observable. For example, the average momentum or the expectation value of the momentum of a particle in the n-th state of the box. The only difference in the procedure to determine the expectation value of position is to replace the position operator with ...
... This process may be performed for any other observable. For example, the average momentum or the expectation value of the momentum of a particle in the n-th state of the box. The only difference in the procedure to determine the expectation value of position is to replace the position operator with ...
Titles and Abstracts
... Joseph Ben Geloun (Perimeter Institute for Theoretical Physics, Canada) Title: Enhanced quantization on the circle Abstract: The quantization scheme introduced by Klauder in [arXiv:1204.2870] is applied to a particle on a circle. We find that the quantum action functional restricted to appropriate ...
... Joseph Ben Geloun (Perimeter Institute for Theoretical Physics, Canada) Title: Enhanced quantization on the circle Abstract: The quantization scheme introduced by Klauder in [arXiv:1204.2870] is applied to a particle on a circle. We find that the quantum action functional restricted to appropriate ...
WP1
... with themselves! What! How? Does a single electron go through both slits (to cause the interference)? How can an electron that causes a localized flash on the absorbing screen go through both slits? Can an electron be in two places at once? Counter common sense. As Feynman said “No one understands q ...
... with themselves! What! How? Does a single electron go through both slits (to cause the interference)? How can an electron that causes a localized flash on the absorbing screen go through both slits? Can an electron be in two places at once? Counter common sense. As Feynman said “No one understands q ...
Operators in Quantum Mechanics
... Extensive account of Operators Historic development of quantum mechanics from classical mechanics The Development of Classical Mechanics Experimental Background for Quantum mecahnics Early Development of Quantum mechanics ...
... Extensive account of Operators Historic development of quantum mechanics from classical mechanics The Development of Classical Mechanics Experimental Background for Quantum mecahnics Early Development of Quantum mechanics ...
Unit 1: Kinematics - Pre University Courses
... (b) Answers may vary. Students should add the following information to their concept maps: Louis de Broglie believed that all entities have wave-like properties but these properties are only significant and measureable for tiny, fast-moving particles like the electron. Erwin Schrödinger imagined el ...
... (b) Answers may vary. Students should add the following information to their concept maps: Louis de Broglie believed that all entities have wave-like properties but these properties are only significant and measureable for tiny, fast-moving particles like the electron. Erwin Schrödinger imagined el ...
Dave Bacon on Quantum Error Correction. Slides in PPT.
... of particular physical implementations physical implementation gate speeds gate accuracies gate costs forms of decoherence decoherence times shuttling speeds shuttling accuracies ...
... of particular physical implementations physical implementation gate speeds gate accuracies gate costs forms of decoherence decoherence times shuttling speeds shuttling accuracies ...
sch4u-quantumtheory
... • In 1924, a French physicist named Louis de Broglie suggested that, like light, electrons could act as both particles and waves. • De Broglie's hypothesis was soon confirmed in experiments that showed electron beams could be diffracted or bent as they passed through a slit much like light could. • ...
... • In 1924, a French physicist named Louis de Broglie suggested that, like light, electrons could act as both particles and waves. • De Broglie's hypothesis was soon confirmed in experiments that showed electron beams could be diffracted or bent as they passed through a slit much like light could. • ...
Atlantis Studies in Mathematical Physics: Theory and Applications
... The aim of this book series is to publish high quality monographs in mathematical physics of both theoretical and applied subjects. The main topics are listed below. We expect the books of this series to reflect the current state of the art of some of the popular ongoing research topics in mathemati ...
... The aim of this book series is to publish high quality monographs in mathematical physics of both theoretical and applied subjects. The main topics are listed below. We expect the books of this series to reflect the current state of the art of some of the popular ongoing research topics in mathemati ...
Wave mechanics and the Schrödinger equation
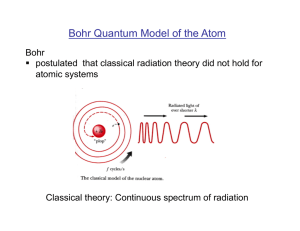
... consideration of a planetary model of the atom. However, a classical theory of electrodynamics would predict that an accelerating charge would radiate energy leading to the eventual collapse of the electron into the nucleus. Moreover, as the electron spirals inwards, the emission would gradually inc ...
... consideration of a planetary model of the atom. However, a classical theory of electrodynamics would predict that an accelerating charge would radiate energy leading to the eventual collapse of the electron into the nucleus. Moreover, as the electron spirals inwards, the emission would gradually inc ...
Chapter 7 Worksheet November 1
... B. The value of l specifies the orientation of the orbital C. The value of l specifies the energy level of the orbital. D. The number of possible l values equals the value of n. ...
... B. The value of l specifies the orientation of the orbital C. The value of l specifies the energy level of the orbital. D. The number of possible l values equals the value of n. ...
Chapter 5
... – Periodic table powerpoint – elements of a group have similar properties – Chapter 2 – elements in a group form similar formulas – Predict the properties of an element by knowing the properties of other elements in the group ...
... – Periodic table powerpoint – elements of a group have similar properties – Chapter 2 – elements in a group form similar formulas – Predict the properties of an element by knowing the properties of other elements in the group ...
What is Light?
... – “I believe that the existence of the classical ‘path’ can be pregnantly formulated as follows: The ‘path’ comes into existence only when we observe it. “ – “In the sharp formulation of the law of causality-- "if we know the present exactly, we can calculate the future"- it is not the conclusion th ...
... – “I believe that the existence of the classical ‘path’ can be pregnantly formulated as follows: The ‘path’ comes into existence only when we observe it. “ – “In the sharp formulation of the law of causality-- "if we know the present exactly, we can calculate the future"- it is not the conclusion th ...