Chapter 7 -- Radiative Corrections: some formal developments Chapter 7:
... W hen it seemed that about hydrogen atom we knew almost everything in 1947 W.E. Lamb and R.C. Retherford decided to check results of Dirac. They used microwaves technique, available from the constructions of radar The Lamb's shift*, a minimal difference in lowest energetic level of the excited hydro ...
... W hen it seemed that about hydrogen atom we knew almost everything in 1947 W.E. Lamb and R.C. Retherford decided to check results of Dirac. They used microwaves technique, available from the constructions of radar The Lamb's shift*, a minimal difference in lowest energetic level of the excited hydro ...
DARLLENWCH Y DARN ISOD AC ATEBWCH Y CWESTIYNAU SY
... the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus. To remedy the stability problem, Bohr modified the ...
... the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus. To remedy the stability problem, Bohr modified the ...
Chapter 8 - Bakersfield College
... photons all of the same frequency and all of whose waves are coherent or exactly in step. 8-12. Quantum Mechanics A. The theory of quantum mechanics was developed by Erwin Schrödinger, Werner Heisenberg, and others during the mid-1920s. B. According to quantum mechanics, the position and momentum of ...
... photons all of the same frequency and all of whose waves are coherent or exactly in step. 8-12. Quantum Mechanics A. The theory of quantum mechanics was developed by Erwin Schrödinger, Werner Heisenberg, and others during the mid-1920s. B. According to quantum mechanics, the position and momentum of ...
Physics 228, Lecture 11 Monday, February 28, 2005 Bohr Model
... on quantum mechanical principles? Didn’t we have good evidence to support what we learned about Newtonian mechanics and Maxwell’s theory of electromagnetism? The same issue came up with spectial relativity, where we “threw out” the fundamental understanding of how coordinate systems were related, ch ...
... on quantum mechanical principles? Didn’t we have good evidence to support what we learned about Newtonian mechanics and Maxwell’s theory of electromagnetism? The same issue came up with spectial relativity, where we “threw out” the fundamental understanding of how coordinate systems were related, ch ...
File
... b. Rutherford c. Democritus d. Einstein 2. Which observation led J.J. Thomson to conclude that negative particles existed within the atom? a. deflection of alpha particles b. atomic absorption spectra c. atomic emission spectra d. the deflection of cathode rays by an electric field e. absorption of ...
... b. Rutherford c. Democritus d. Einstein 2. Which observation led J.J. Thomson to conclude that negative particles existed within the atom? a. deflection of alpha particles b. atomic absorption spectra c. atomic emission spectra d. the deflection of cathode rays by an electric field e. absorption of ...
Waves - Valdosta State University
... • Evidence for the particle nature of light • Light shines on a metal surface • If above a threshold frequency---electrons ejected • If below the threshold frequency---no electrons ejected (no matter how intense the light) • Number of electrons ejected depends on light intensity • Recall light comes ...
... • Evidence for the particle nature of light • Light shines on a metal surface • If above a threshold frequency---electrons ejected • If below the threshold frequency---no electrons ejected (no matter how intense the light) • Number of electrons ejected depends on light intensity • Recall light comes ...
The Modern Atomic Model
... (like planets) – often called “Planetary Model”. • Experiments greater than 1 electron systems failed to reproduce this motion. ...
... (like planets) – often called “Planetary Model”. • Experiments greater than 1 electron systems failed to reproduce this motion. ...
Quantum Mechanics
... kind of particle. Particles are just localized bunches of energy carried by the fields. Particles can appear and disappear spontaneously from the fields. Perhaps the universe appeared in just this way. ...
... kind of particle. Particles are just localized bunches of energy carried by the fields. Particles can appear and disappear spontaneously from the fields. Perhaps the universe appeared in just this way. ...
About Heisenberg`s Uncertainty Principle
... contradiction, which is detected by comparing the results of observations of the atomic object obtained using different experimental setups. Such empirical evidence indicates the presence of a new type of relations which have no analogues in classical physics, and is convenient to define the term co ...
... contradiction, which is detected by comparing the results of observations of the atomic object obtained using different experimental setups. Such empirical evidence indicates the presence of a new type of relations which have no analogues in classical physics, and is convenient to define the term co ...
PHYSICS 100
... Thin film interference occurs when light incident on a thin film is partially reflected at the top surface and partially transmitted through the film. The transmitted ray reflects off the bottom of the film and travels up and through the top of the film. The two reflected rays have a path length dif ...
... Thin film interference occurs when light incident on a thin film is partially reflected at the top surface and partially transmitted through the film. The transmitted ray reflects off the bottom of the film and travels up and through the top of the film. The two reflected rays have a path length dif ...
Midterm Exam No. 03 (Spring 2015) PHYS 520B: Electromagnetic Theory
... to solve the differential equation in Eq. (1) to find the position x(t) and velocity v(t) as a function of time. Use ω = qB/m. (b) In particular, prove that the particle takes a path along a cycloid. That is, the particle moves as though it were a spot on the rim of a wheel rolling along the xaxis. ...
... to solve the differential equation in Eq. (1) to find the position x(t) and velocity v(t) as a function of time. Use ω = qB/m. (b) In particular, prove that the particle takes a path along a cycloid. That is, the particle moves as though it were a spot on the rim of a wheel rolling along the xaxis. ...
e - Purdue Physics - Purdue University
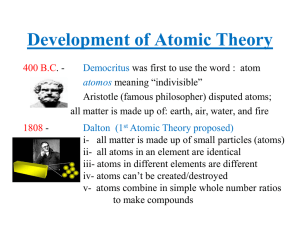
... The results of many experiments collectively suggest that all matter is made up of small, indivisible units which have a unique identity. Study of chemistry suggests a number of elementary ...
... The results of many experiments collectively suggest that all matter is made up of small, indivisible units which have a unique identity. Study of chemistry suggests a number of elementary ...
IntroQuantumNuclearp..
... wavefunction equation (ψ) model Predicted behavior of e- in space and time – think of it as predicting where and when an e- based on probability* If you map out these likely locations over time, you would see a “cloud” of possible locations around the nucleus* |ψ|2 is proportional to the probability ...
... wavefunction equation (ψ) model Predicted behavior of e- in space and time – think of it as predicting where and when an e- based on probability* If you map out these likely locations over time, you would see a “cloud” of possible locations around the nucleus* |ψ|2 is proportional to the probability ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.