LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 10. Write down the Dirac matrices in terms of the (2x2) Pauli spin matrices and unit matrix PART B ...
... 10. Write down the Dirac matrices in terms of the (2x2) Pauli spin matrices and unit matrix PART B ...
Chapter 7 NotesAA
... know both the position and momentum of a particle at a given time. In layman’s terms: The more precisely we know a particles position the less we know about its momentum and vice versa. ...
... know both the position and momentum of a particle at a given time. In layman’s terms: The more precisely we know a particles position the less we know about its momentum and vice versa. ...
REVIEW OF WAVE MECHANICS
... However this means that in general measurements are not passive and profoundly disturb the state of a system. If the initial wave function of a system is described as a linear superposition of the eigenfunctions before the measurement, after the measurement it has been “reduced” or “collapsed” to on ...
... However this means that in general measurements are not passive and profoundly disturb the state of a system. If the initial wave function of a system is described as a linear superposition of the eigenfunctions before the measurement, after the measurement it has been “reduced” or “collapsed” to on ...
s - Dl4a.org
... slit 1 or slit 2 on its way to the backstop • If so, then for those that pass through slit 1, say, it cannot matter whether slit 2 is open or closed (and vice versa) • The total distribution of electrons at the backstop is thus the sum of those passing through slit 1 with those passing through slit ...
... slit 1 or slit 2 on its way to the backstop • If so, then for those that pass through slit 1, say, it cannot matter whether slit 2 is open or closed (and vice versa) • The total distribution of electrons at the backstop is thus the sum of those passing through slit 1 with those passing through slit ...
Quantum Theory and Molecular Energy
... What is the wavefunction? The Born Interpretation of the Wavefunction: It is a mathematical (sometimes imaginary) function of the coordinate(s). The square of the wavefunction is interpreted as being proportional to the probability of the particle(s) being a particular value of the coordinates. In 1 ...
... What is the wavefunction? The Born Interpretation of the Wavefunction: It is a mathematical (sometimes imaginary) function of the coordinate(s). The square of the wavefunction is interpreted as being proportional to the probability of the particle(s) being a particular value of the coordinates. In 1 ...
Introduction Slides
... of particles photons, of energy E h The kinetic energy of the emitted electrons is the energy left over after the electron has been “lifted” over the work function barrier ...
... of particles photons, of energy E h The kinetic energy of the emitted electrons is the energy left over after the electron has been “lifted” over the work function barrier ...
Introduction to Chemistry
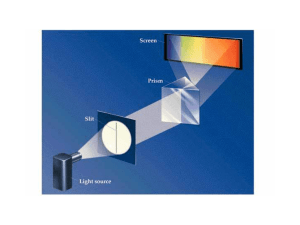
... Learning Objectives (from Zumdahl Resource Guide): (3-4 days lecture/discussion) To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffract ...
... Learning Objectives (from Zumdahl Resource Guide): (3-4 days lecture/discussion) To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffract ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.