Chapter 31 Quantum Mechanics and Atomic Physics
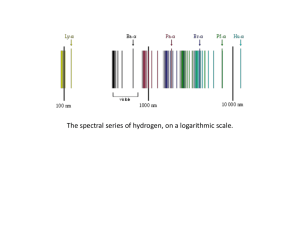
... Line spectrum A line spectrum is a series of discrete electromagnetic wavelengths emitted by the atoms of a low-pressure gas that is subjected to a sufficiently high potential difference. Certain groups of discrete wavelengths are referred to as "series." The line spectrum of atomic hydrogen include ...
... Line spectrum A line spectrum is a series of discrete electromagnetic wavelengths emitted by the atoms of a low-pressure gas that is subjected to a sufficiently high potential difference. Certain groups of discrete wavelengths are referred to as "series." The line spectrum of atomic hydrogen include ...
1 – Foundations of Quantum Theory
... of the problem to predict the physical application • His math matched the experimental results perfectly (Now that’s good Physics!!!) • The conclusion of the research (not his conclusion) was interpreted to be that the energy could only be released in quantised (packet) form now referred to as a pho ...
... of the problem to predict the physical application • His math matched the experimental results perfectly (Now that’s good Physics!!!) • The conclusion of the research (not his conclusion) was interpreted to be that the energy could only be released in quantised (packet) form now referred to as a pho ...
Sections 6.3-6.5
... • Electrons move in certain, specific, circular orbitals • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
... • Electrons move in certain, specific, circular orbitals • Smaller orbit = lower energy level • Assigned the allowable electron orbitals the principle quantum number, n. • 1st orbit= lowest energy: n=1 • 2nd orbit= 2nd lowest energy: n=2 ...
Book Reviews
... Norris quotes with approval William Alston’ s characterization of the alethic conception of realism, which is the conception advocated by Norris; the alethic conception ª implies that (almost always) what confers a truth-value on a statement is something independent of the cognitive-linguistic going ...
... Norris quotes with approval William Alston’ s characterization of the alethic conception of realism, which is the conception advocated by Norris; the alethic conception ª implies that (almost always) what confers a truth-value on a statement is something independent of the cognitive-linguistic going ...
Sample Exam 3
... 17. You may have noticed that a bound electron (q = −e, m = me ) orbiting a proton (q = +e, m = mp ) in the Bohr model atom obeys the following relation: 2 KEn = –PEn . (a) If an excited electron orbits a proton at a distance of 1.9044 nm, what is the potential energy of this electron in eV? (b) Wha ...
... 17. You may have noticed that a bound electron (q = −e, m = me ) orbiting a proton (q = +e, m = mp ) in the Bohr model atom obeys the following relation: 2 KEn = –PEn . (a) If an excited electron orbits a proton at a distance of 1.9044 nm, what is the potential energy of this electron in eV? (b) Wha ...
Questions for learning Quantum Mechanics of FYSA21
... Ulf von Barth, Tomas Brage and Peter Samuelsson October 27, 2014 ...
... Ulf von Barth, Tomas Brage and Peter Samuelsson October 27, 2014 ...
Student Text, pp. 650-653
... mechanics and entered the new, uncharted world of quantum mechanics, many phenomena were discovered that were both strange and difficult to visualize. In classical mechanics, objects we identify as particles always behave like particles, and wave phenomena always exhibit pure wave properties. But th ...
... mechanics and entered the new, uncharted world of quantum mechanics, many phenomena were discovered that were both strange and difficult to visualize. In classical mechanics, objects we identify as particles always behave like particles, and wave phenomena always exhibit pure wave properties. But th ...
Rutherford–Bohr model
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
Document
... exact position and the exact momentum of an object at the same time. Applying this concept to the electron we realize that in order to get a fix on an electron's position at any time, we would alter its momentum. Any attempt to study the velocity of an electron will alter its position. This concept, ...
... exact position and the exact momentum of an object at the same time. Applying this concept to the electron we realize that in order to get a fix on an electron's position at any time, we would alter its momentum. Any attempt to study the velocity of an electron will alter its position. This concept, ...
quantum - UCI Physics and Astronomy
... In the 20th century, study of atomic systems required a fundamental revision of these classical ideas about physical objects. 1. Light waves exhibited particle like properties – phenomena called photo-electric effect in which light impinging on certain metals cause instanteous emission of electrons ...
... In the 20th century, study of atomic systems required a fundamental revision of these classical ideas about physical objects. 1. Light waves exhibited particle like properties – phenomena called photo-electric effect in which light impinging on certain metals cause instanteous emission of electrons ...
Epistemological Foun.. - University of Manitoba
... Indeterminacy has proven to be one of the most radical innovations in musical composition of the twentieth century. If extensively applied, it eliminates style. It can remove all consistently distinctive features form the work of a composer and thus put his accomplishments at a distance from the lis ...
... Indeterminacy has proven to be one of the most radical innovations in musical composition of the twentieth century. If extensively applied, it eliminates style. It can remove all consistently distinctive features form the work of a composer and thus put his accomplishments at a distance from the lis ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 15. Write down the Dirac matrices in terms of Pauli spin matrices and establish their anticommuting properties. PART C (4 x 12 1/2m = 50m) Answer any FOUR questions 16. (a) Discuss the work-energy theorem in relativity. (b) The coordinates of event A are ( x A, 0, 0, t A) and the coordinates of even ...
... 15. Write down the Dirac matrices in terms of Pauli spin matrices and establish their anticommuting properties. PART C (4 x 12 1/2m = 50m) Answer any FOUR questions 16. (a) Discuss the work-energy theorem in relativity. (b) The coordinates of event A are ( x A, 0, 0, t A) and the coordinates of even ...
The interpretation of the Einstein-Rupp experiments and their
... the back in 2 can not produce any sharp fringes, etc. 9 This idea of Lorentz essentially resurfaced in Einstein’s Wire Grid Experiment, where the cutting up into the “two wave trains 1 and 2” of the interference field would occur because of the grid. If in the Wire Grid Experiment a variability in t ...
... the back in 2 can not produce any sharp fringes, etc. 9 This idea of Lorentz essentially resurfaced in Einstein’s Wire Grid Experiment, where the cutting up into the “two wave trains 1 and 2” of the interference field would occur because of the grid. If in the Wire Grid Experiment a variability in t ...
Chapter 30: Quantum Physics Chapter 31: Atomic Physics Chapter
... If energy is quantized, as suggested by Planck, the amount of energy for even a single high-frequency photon can be arbitrarily large. The finite energy in a blackbody simply can’t produce such high-frequency photons, and therefore the infinite energy implied by the “ultraviolet catastrophe” cannot ...
... If energy is quantized, as suggested by Planck, the amount of energy for even a single high-frequency photon can be arbitrarily large. The finite energy in a blackbody simply can’t produce such high-frequency photons, and therefore the infinite energy implied by the “ultraviolet catastrophe” cannot ...
Exam 3 review problems from the course text, Serway and Jewett
... 36.28 Thin lenses and focal length 36.32 Magnification of images 36.34 Virtual Image ...
... 36.28 Thin lenses and focal length 36.32 Magnification of images 36.34 Virtual Image ...
Lecture 24 (7.1-7.2)
... The particle nature of light • Blackbody radiation – light emitted from solid objects heated to incandescence – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained th ...
... The particle nature of light • Blackbody radiation – light emitted from solid objects heated to incandescence – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained th ...
Q15
... Placing the particle in a box restricts the possible wavelengths as only waves which are zero at the walls are allowed. This leads to quantization of the wavelength and this, in turn, restricts the possible energy that the particle can have. The longest wavelength now corresponds to the wave shown ...
... Placing the particle in a box restricts the possible wavelengths as only waves which are zero at the walls are allowed. This leads to quantization of the wavelength and this, in turn, restricts the possible energy that the particle can have. The longest wavelength now corresponds to the wave shown ...
Q.M3 Home work 9 Due date 3.1.15 1
... A coherent state is the specific quantum state of the quantum harmonic oscillator whose dynamics most closely resembles the oscillating behaviour of a classical harmonic oscillator. Further, in contrast to the energy eigenstates of the system, the time evolution of a coherent state is concentrated a ...
... A coherent state is the specific quantum state of the quantum harmonic oscillator whose dynamics most closely resembles the oscillating behaviour of a classical harmonic oscillator. Further, in contrast to the energy eigenstates of the system, the time evolution of a coherent state is concentrated a ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.