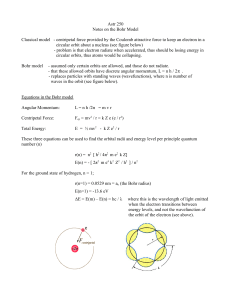
Historical Introduction to the Elementary Particles
... deflected by a magnet. This suggested that they carried electric charge; • 2. in fact, the direction of the curvature required that the charge be negative. • 3. It seemed, therefore, that these were not rays at all, but rather streams of particles. • 4. By passing the beam through electric and magne ...
... deflected by a magnet. This suggested that they carried electric charge; • 2. in fact, the direction of the curvature required that the charge be negative. • 3. It seemed, therefore, that these were not rays at all, but rather streams of particles. • 4. By passing the beam through electric and magne ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 1. Distinguish between timelike and spacelike intervals. 2. Write down the Lorentz transformation equations between the proper velocities in two inertial frames for a boost along the common x-axis. 3. How does charge density transform under Lorentz transformation? 4. What is 4-potential in relativis ...
... 1. Distinguish between timelike and spacelike intervals. 2. Write down the Lorentz transformation equations between the proper velocities in two inertial frames for a boost along the common x-axis. 3. How does charge density transform under Lorentz transformation? 4. What is 4-potential in relativis ...
Measurement in Quantum Mechanics
... Crucial to the thinking of the Copenhagen school, was that the only sensible questions were those which have, at least in principle, and operational, experimental meaning. So the notion of an electron’s simultaneous momentum and position, for example, was not meaningful. The basic ingredients in the ...
... Crucial to the thinking of the Copenhagen school, was that the only sensible questions were those which have, at least in principle, and operational, experimental meaning. So the notion of an electron’s simultaneous momentum and position, for example, was not meaningful. The basic ingredients in the ...
qp2
... fourth component to an electron's wave function… its spin. Hence it was theorized that 2 electrons could exist in the same quantum state, also known as orbital, assuming only when they spin that they spin in opposite directions. Hence electrons keep their distance and lead to atomic sizes as we see. ...
... fourth component to an electron's wave function… its spin. Hence it was theorized that 2 electrons could exist in the same quantum state, also known as orbital, assuming only when they spin that they spin in opposite directions. Hence electrons keep their distance and lead to atomic sizes as we see. ...
Key Concepts for Exam #2
... light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons increases and the number of electrons remains constant. If the frequency of the light is below th ...
... light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons increases and the number of electrons remains constant. If the frequency of the light is below th ...
Eighth International Conference on Geometry, Integrability and Quantization
... In the nineteen fifties of previous century, Destouches [1, 2] developed his functional quantum theory as a generalization of de Broglie’s theory. His basic idea was that elementary particles need not be pointlike. Being extended and non rigid is a better conception. Rather than conceiving the parti ...
... In the nineteen fifties of previous century, Destouches [1, 2] developed his functional quantum theory as a generalization of de Broglie’s theory. His basic idea was that elementary particles need not be pointlike. Being extended and non rigid is a better conception. Rather than conceiving the parti ...
lec21
... In the case of sound waves in air, it is air molecules that are doing the “waving”. What is “waving” in the case of light? a) Electrons. b) Protons. c) Both a and b. d) Electric and magnetic fields. e) None of the above. ...
... In the case of sound waves in air, it is air molecules that are doing the “waving”. What is “waving” in the case of light? a) Electrons. b) Protons. c) Both a and b. d) Electric and magnetic fields. e) None of the above. ...
Slide 1
... In the case of sound waves in air, it is air molecules that are doing the “waving”. What is “waving” in the case of light? a) Electrons. b) Protons. c) Both a and b. d) Electric and magnetic fields. e) None of the above. ...
... In the case of sound waves in air, it is air molecules that are doing the “waving”. What is “waving” in the case of light? a) Electrons. b) Protons. c) Both a and b. d) Electric and magnetic fields. e) None of the above. ...
Lecture6.QM.to.Lagrangian.Densities
... and the Euler-Lagrange equations give F = ma In quantum field theory, the Euler-Lagrange equations give the particle wave function! ...
... and the Euler-Lagrange equations give F = ma In quantum field theory, the Euler-Lagrange equations give the particle wave function! ...