T The quantum and classical properties of spins on surfaces
... magnetic atoms on a surface behaves similar to a classical magnetic particle: it’s magnetization points along an easyaxis direction in space and magnetization reversal requires sufficient thermal energy to overcome a barrier. In this talk we will discuss how many atoms it takes to create such create ...
... magnetic atoms on a surface behaves similar to a classical magnetic particle: it’s magnetization points along an easyaxis direction in space and magnetization reversal requires sufficient thermal energy to overcome a barrier. In this talk we will discuss how many atoms it takes to create such create ...
Quantum Mechanics in a Nutshell
... – The solution corresponding to the lowest Ei is the ground state – Ei is a scalar while ψi is a vector – The ψis are orthonormal, i.e., Int{ψi(r)ψj(r)d3r} = ij – If H is hermitian, Ei are all real (although ψi are complex) – Can be cast as a differential equation (Schrodinger) or a ...
... – The solution corresponding to the lowest Ei is the ground state – Ei is a scalar while ψi is a vector – The ψis are orthonormal, i.e., Int{ψi(r)ψj(r)d3r} = ij – If H is hermitian, Ei are all real (although ψi are complex) – Can be cast as a differential equation (Schrodinger) or a ...
Lecture 5
... tells you the probability that a measurement of the energy would yield the value En. Only the values En can be obtained as results of the energy measurements. The sum of all these probabilities will be, of course, 1. (see proof in the textbook) ...
... tells you the probability that a measurement of the energy would yield the value En. Only the values En can be obtained as results of the energy measurements. The sum of all these probabilities will be, of course, 1. (see proof in the textbook) ...
wave function
... of quantization to electromagnetic waves All electromagnetic radiation can be considered a stream of quanta, now called photons A photon of incident light gives all its energy hƒ to a single electron in the metal ...
... of quantization to electromagnetic waves All electromagnetic radiation can be considered a stream of quanta, now called photons A photon of incident light gives all its energy hƒ to a single electron in the metal ...
WHY STUDY QUANTUM CHEMISTRY? Physical Chemisty can be
... mechanics and quantum hypotheses. It was not rigorously derived from first principles. It was only accurate for oneelectron atoms or ions (5% in error for helium) THE FORMULATION OF QUANTUM MECHANICS 1926 - Schrödinger formulated quantum (or wave) mechanics to describe wavelike behavior & energy qua ...
... mechanics and quantum hypotheses. It was not rigorously derived from first principles. It was only accurate for oneelectron atoms or ions (5% in error for helium) THE FORMULATION OF QUANTUM MECHANICS 1926 - Schrödinger formulated quantum (or wave) mechanics to describe wavelike behavior & energy qua ...
Quantum Mechanics: PHL555 Tutorial 2
... ( s1x , s1z etc. )while the observer B measures the spin component of the other particle. Suppose the system is known to be in the spin-singlet state, that is stotal 0 . (a) What is the probability of for observer A to ...
... ( s1x , s1z etc. )while the observer B measures the spin component of the other particle. Suppose the system is known to be in the spin-singlet state, that is stotal 0 . (a) What is the probability of for observer A to ...
Particle acceleration by electric field in an 3D RCS
... Particle acceleration by electric field in an 3D RCS Valentina Zharkova and Mykola Gordovskyy ...
... Particle acceleration by electric field in an 3D RCS Valentina Zharkova and Mykola Gordovskyy ...
CHAPTER 4 TEST REVIEW GUIDE
... 11. Read electron configurations (all three styles) and identify what each of the symbols represents (ex. 1S22S1…). Know how to use the Periodic Table to produce and read electron configurations. 12. Write electron configurations for selected elements in each of the three styles of notation (orbita ...
... 11. Read electron configurations (all three styles) and identify what each of the symbols represents (ex. 1S22S1…). Know how to use the Periodic Table to produce and read electron configurations. 12. Write electron configurations for selected elements in each of the three styles of notation (orbita ...
Prof. Bertrand Reulet, Université de Sherbrooke, Canada Talk: 23. May 2014
... > fluctuations of the electrical current, a phenomenon commonly referred > to as "noise". In classical physics, the variance of these > fluctuations is simply proportional to the temperature. In a tiny > device placed at very low temperature, electrons can no longer be > considered as classical part ...
... > fluctuations of the electrical current, a phenomenon commonly referred > to as "noise". In classical physics, the variance of these > fluctuations is simply proportional to the temperature. In a tiny > device placed at very low temperature, electrons can no longer be > considered as classical part ...
PerturbationTheory
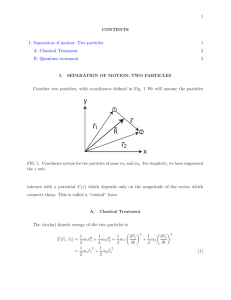
... Pb nucleus so that they interact through the nuclear force (in addition to the Coulomb force that acts when they are further apart) the Rutherford formula ...
... Pb nucleus so that they interact through the nuclear force (in addition to the Coulomb force that acts when they are further apart) the Rutherford formula ...
There are a total of n subshells, each specified by an
... Moving charges give rise to magnetic fields, which will then interact. Since the magnetic moments never align with the “z-axis” the torque is never zero. ...
... Moving charges give rise to magnetic fields, which will then interact. Since the magnetic moments never align with the “z-axis” the torque is never zero. ...
Physics Qualifier Part I—Spring 2010 7-Minute Questions α
... where ωpe is the electron plasma frequency, and γ describes the dissipative processes, γ ≪ ω. Assume that the permeability of the plasma is µ ≈ µ0 , and that ωpe ≪ ω. Estimate the distance such a wave can propagate inside the plasma before it gets significantly attenuated. 7. It is well-known that t ...
... where ωpe is the electron plasma frequency, and γ describes the dissipative processes, γ ≪ ω. Assume that the permeability of the plasma is µ ≈ µ0 , and that ωpe ≪ ω. Estimate the distance such a wave can propagate inside the plasma before it gets significantly attenuated. 7. It is well-known that t ...
Quantum numbers
... Theorizing that the electron acts like a wave, and has a wave function That represents the x, y and z coordinates of the electron. A specific wave function is often called an orbital. ...
... Theorizing that the electron acts like a wave, and has a wave function That represents the x, y and z coordinates of the electron. A specific wave function is often called an orbital. ...























