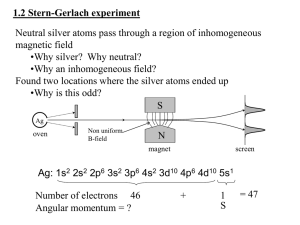
1 The Photoelectric Effect 2 Line Spectra and Energy Levels
... In Bohr’s hypothesis, the allowed values of angular momentum L for an electron in a circular orbit are given by L = mνr − n ...
... In Bohr’s hypothesis, the allowed values of angular momentum L for an electron in a circular orbit are given by L = mνr − n ...
Electron Notes
... • One experiment involved the photoelectric effect, which refers to the emission of electrons from a metal when light shines on the metal. • This involved the frequency of the light. It was found that light was a form of energy that could knock an electron loose from a metal. ...
... • One experiment involved the photoelectric effect, which refers to the emission of electrons from a metal when light shines on the metal. • This involved the frequency of the light. It was found that light was a form of energy that could knock an electron loose from a metal. ...
22-2 Electromagnetic Waves and the Electromagnetic
... Our eyes are sensitive to electromagnetic (EM) waves that have wavelengths in the visible spectrum, between 400 and 700 nm, but our bodies can be affected by EM waves in other ways, too. We have some sensors on the backs of our hands, in particular, that are sensitive to infrared radiation, which we ...
... Our eyes are sensitive to electromagnetic (EM) waves that have wavelengths in the visible spectrum, between 400 and 700 nm, but our bodies can be affected by EM waves in other ways, too. We have some sensors on the backs of our hands, in particular, that are sensitive to infrared radiation, which we ...
2 - Physics at Oregon State University
... • Also called “spin”, or spin angular momentum, or S • It’s a “degree of freedom”, or quantum number: a “state” the particle has • Does interact with magnetic fields like L, but not continuous! • NOT a physical rotation • INTRINSIC property – like charge and rest mass! We have no model for what “mak ...
... • Also called “spin”, or spin angular momentum, or S • It’s a “degree of freedom”, or quantum number: a “state” the particle has • Does interact with magnetic fields like L, but not continuous! • NOT a physical rotation • INTRINSIC property – like charge and rest mass! We have no model for what “mak ...
Walker3_Lecture_Ch30
... This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permit ...
... This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permit ...
uncertainty, atom
... charge, and accelerating charges give off EM radiation (like an antenna), thus giving off energy. The electron would gradually lose all its energy. That doesn’t happen -- atoms are stable. ...
... charge, and accelerating charges give off EM radiation (like an antenna), thus giving off energy. The electron would gradually lose all its energy. That doesn’t happen -- atoms are stable. ...
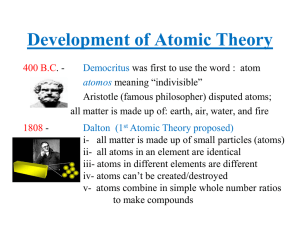
Development of Atomic Theory
... - electrons travel in these orbits without losing energy (stationary state) but could gain energy and jump into a higher orbit (excited or transition state) or could lose energy and fall to a lower orbit, or the lowest orbit (ground state) ...
... - electrons travel in these orbits without losing energy (stationary state) but could gain energy and jump into a higher orbit (excited or transition state) or could lose energy and fall to a lower orbit, or the lowest orbit (ground state) ...
The electron! Speed and energy notes
... electrons can return to ground state by giving off the energy as a color of light, called photons of light. ...
... electrons can return to ground state by giving off the energy as a color of light, called photons of light. ...
REVIEW OF WAVE MECHANICS
... Q n q n n , where Q is an operator (e.g. the Hamiltonian on the left hand side of Schrodinger’s equations), and qn and n respectively are the eigenvalues and eigenfunctions of this operator labelled by quantum number(s) n(lm...). Then a measurement of the quantity represented by the operator ...
... Q n q n n , where Q is an operator (e.g. the Hamiltonian on the left hand side of Schrodinger’s equations), and qn and n respectively are the eigenvalues and eigenfunctions of this operator labelled by quantum number(s) n(lm...). Then a measurement of the quantity represented by the operator ...