Document
... Even with a qualitative treatment it is possible to see how the Feynman diagram picture agrees with observed reactions. Aim: compare rates of e e g g and e e g g g Start e e g g and consider possible contributing diagrams. Use the simplest possible diagrams (leading or ...
... Even with a qualitative treatment it is possible to see how the Feynman diagram picture agrees with observed reactions. Aim: compare rates of e e g g and e e g g g Start e e g g and consider possible contributing diagrams. Use the simplest possible diagrams (leading or ...
Chemistry—Chapter 13: Electrons in Atoms
... 4. Write a complete electron configuration of each atom, including orbital notations. Give the quantum numbers for the highest energy electron in each atom a. hydrogen b. barium c. bromine d. sulfur e. krypton f. arsenic g. vanadium ...
... 4. Write a complete electron configuration of each atom, including orbital notations. Give the quantum numbers for the highest energy electron in each atom a. hydrogen b. barium c. bromine d. sulfur e. krypton f. arsenic g. vanadium ...
in-class worksheet
... Name___________________________________________________ QUANTUM MECHANICAL MODEL OF THE ATOM Contributors to the quantum mechanical model in mid-1920s: Louis deBroglie Erwin Schrödinger Werner Heisenberg Schrödinger – treat e– as a wave Schrödinger equation: Ĥ = E solve to get wave functions, whic ...
... Name___________________________________________________ QUANTUM MECHANICAL MODEL OF THE ATOM Contributors to the quantum mechanical model in mid-1920s: Louis deBroglie Erwin Schrödinger Werner Heisenberg Schrödinger – treat e– as a wave Schrödinger equation: Ĥ = E solve to get wave functions, whic ...
January 2010
... A uniform rigid slab of mass M is supported by two rapidly counter-rotating parallel horizontal rollers, with axes a distance d apart, with surfaces that brush past the slab in the directions shown in the figure. The coefficient of kinetic friction between each roller and the slab is µk . At time t ...
... A uniform rigid slab of mass M is supported by two rapidly counter-rotating parallel horizontal rollers, with axes a distance d apart, with surfaces that brush past the slab in the directions shown in the figure. The coefficient of kinetic friction between each roller and the slab is µk . At time t ...
atomsagain
... The full wave function is given by: •n, the principal quantum number, tells you the energy n 1, 2,3, •l tells you the total angular momentum l 0,1, 2, , n 1 •m tells you the angular momentum around the z-axis An electron in hydrogen is in the state 3,2,1 m l , l 1, , l •What is the wav ...
... The full wave function is given by: •n, the principal quantum number, tells you the energy n 1, 2,3, •l tells you the total angular momentum l 0,1, 2, , n 1 •m tells you the angular momentum around the z-axis An electron in hydrogen is in the state 3,2,1 m l , l 1, , l •What is the wav ...
E k
... SOP for Band Structure 1. Think a Photoelectric Effect Experiment by Einstein Potential Energy of Interest 2. Degree of Freedom (DOF) and Kinetic Energy 3. Combine Newton’s Mechanisms and De Broglie’s Hypothesis ...
... SOP for Band Structure 1. Think a Photoelectric Effect Experiment by Einstein Potential Energy of Interest 2. Degree of Freedom (DOF) and Kinetic Energy 3. Combine Newton’s Mechanisms and De Broglie’s Hypothesis ...
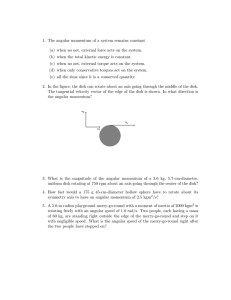
1. The angular momentum of a system remains constant (a) when no
... (a) when no net, external force acts on the system. (b) when the total kinetic energy is constant. (c) when no net, external torque acts on the system. (d) when only conservative torques act on the system. (e) all the time since it is a conserved quantity. 2. In the figure, the disk can rotate about ...
... (a) when no net, external force acts on the system. (b) when the total kinetic energy is constant. (c) when no net, external torque acts on the system. (d) when only conservative torques act on the system. (e) all the time since it is a conserved quantity. 2. In the figure, the disk can rotate about ...
PDF (Size: 3.8M)
... discovered X-rays. He also determined the properties of X-rays and got a Nobel prize in 1901 for this discovery. Later scientists studied the diffraction of X-rays by crystals and Bragg obtained the spectrum of X-ray by making electrons collide with solid substances. In 1896, Becquerel studied the t ...
... discovered X-rays. He also determined the properties of X-rays and got a Nobel prize in 1901 for this discovery. Later scientists studied the diffraction of X-rays by crystals and Bragg obtained the spectrum of X-ray by making electrons collide with solid substances. In 1896, Becquerel studied the t ...
Chapter_5
... • Rutherford’s explanation of the atom was found to be incomplete. • Questions remained as to where the electrons in an atom were located and why the nucleus did not pull them into itself. ...
... • Rutherford’s explanation of the atom was found to be incomplete. • Questions remained as to where the electrons in an atom were located and why the nucleus did not pull them into itself. ...
Honors Freshman Physics Second Semester Final Exam Review
... inverse square laws electrons, protons, atoms elements ions isotopes electric field definition electric field direction gravitational vs. electric force Hooke’s Law displacement/amplitude equilibrium amplitude frequency period wavelength wave velocity wave interference superposition fixed end wave r ...
... inverse square laws electrons, protons, atoms elements ions isotopes electric field definition electric field direction gravitational vs. electric force Hooke’s Law displacement/amplitude equilibrium amplitude frequency period wavelength wave velocity wave interference superposition fixed end wave r ...
1 Repetition on Maxwell`s Equations and Electromag
... Classical electrodynamic was essentially developed in the 19th century by Henry Cavendish (law of forces between electrical charges 1771), Charles Augustin de Coulomb, Hans Christian Ørsted, André Marie Ampère, Michael Faraday, James Clerk Maxwell (dynamical theory of the electromagnetic field 186 ...
... Classical electrodynamic was essentially developed in the 19th century by Henry Cavendish (law of forces between electrical charges 1771), Charles Augustin de Coulomb, Hans Christian Ørsted, André Marie Ampère, Michael Faraday, James Clerk Maxwell (dynamical theory of the electromagnetic field 186 ...