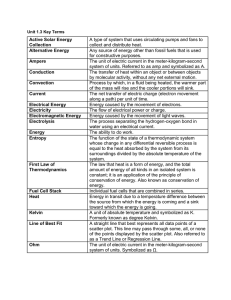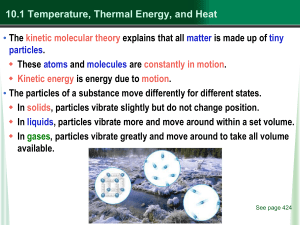
state of matter - Mayfield City Schools
... substance to a higher-temperature substance (You can’t break even; you can’t return to the same energy state because entropy always increases). -When heat flow is spontaneous (without the assistance of external work), the direction of the flow is always from hot to cold. Heat can be made to flow the ...
... substance to a higher-temperature substance (You can’t break even; you can’t return to the same energy state because entropy always increases). -When heat flow is spontaneous (without the assistance of external work), the direction of the flow is always from hot to cold. Heat can be made to flow the ...
doc - University of Colorado Boulder
... from a human being consuming 2,500 kilo-calories (or roughly 10,000,000 Joules per day, assuming the human being is at thermal steady state (doesn’t grow and doesn’t warm up). Q = E / t = ________________________________________ ...
... from a human being consuming 2,500 kilo-calories (or roughly 10,000,000 Joules per day, assuming the human being is at thermal steady state (doesn’t grow and doesn’t warm up). Q = E / t = ________________________________________ ...
Heat Transfer LAB
... 4. What type of heat transfer do the following best describe: conduction, convection or radiation _________________________ a. A bar of chocolate left in the sun begins to melt. _________________________ b. An egg is frying in a pan on a hot electric stove. _________________________ c. Air above a w ...
... 4. What type of heat transfer do the following best describe: conduction, convection or radiation _________________________ a. A bar of chocolate left in the sun begins to melt. _________________________ b. An egg is frying in a pan on a hot electric stove. _________________________ c. Air above a w ...
Title - Iowa State University
... 9: If 20.0 g of solid NaOH are added to 1000 mL of a solution containing 0.500 moles of HCl, the temperature of the solution rises 6.9oC. Assuming that the total solution mass is 1000 g and the specific heat of the solution is 4.184 J/goC, calculate the heat released by this reaction. Then calculate ...
... 9: If 20.0 g of solid NaOH are added to 1000 mL of a solution containing 0.500 moles of HCl, the temperature of the solution rises 6.9oC. Assuming that the total solution mass is 1000 g and the specific heat of the solution is 4.184 J/goC, calculate the heat released by this reaction. Then calculate ...
THERMODYNAMICS - FSU High Energy Physics
... moderating influence on climate some values of specific heat capacity: aluminum 0.21 clay ...
... moderating influence on climate some values of specific heat capacity: aluminum 0.21 clay ...
Ch. 15 - UCSB Physics
... • Human senses can be deceiving • On a cold day: iron railings feel colder than wooden fences, but both have the same T • How can we define T ? • Look for macroscopic changes in a system when heat is added to it ...
... • Human senses can be deceiving • On a cold day: iron railings feel colder than wooden fences, but both have the same T • How can we define T ? • Look for macroscopic changes in a system when heat is added to it ...
Temperature Conversions
... 4. A 400g glass coffee cup is at room temperature, 20.0ºC. It is then plunged into hot dishwater, 80.0ºC. If the temperature of the cup reaches that of the dishwater, how much heat does the cup absorb? Assume the mass of the dishwater is large enough so its temperature doesn’t change appreciably. ...
... 4. A 400g glass coffee cup is at room temperature, 20.0ºC. It is then plunged into hot dishwater, 80.0ºC. If the temperature of the cup reaches that of the dishwater, how much heat does the cup absorb? Assume the mass of the dishwater is large enough so its temperature doesn’t change appreciably. ...
Chapter 9 and 10
... 1st Law: Whenever heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred 2nd Law: Heat never spontaneously flows from a cold substance to a hot substance 3rd Law: No system can reach absolute zero 7. What is a change of phase, or state? Is this a ...
... 1st Law: Whenever heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred 2nd Law: Heat never spontaneously flows from a cold substance to a hot substance 3rd Law: No system can reach absolute zero 7. What is a change of phase, or state? Is this a ...
Period 4 Activity Sheet: Transfer of Thermal Energy
... d) Temperature Scales Your instructor will discuss Fahrenheit, Celsius and Kelvin temperature scales. 1) Examine a thermometer with both Fahrenheit and Celsius scales. On the Celsius scale, how many degrees are between the freezing point and the boiling point of water? ___________ 2) On the Fahrenhe ...
... d) Temperature Scales Your instructor will discuss Fahrenheit, Celsius and Kelvin temperature scales. 1) Examine a thermometer with both Fahrenheit and Celsius scales. On the Celsius scale, how many degrees are between the freezing point and the boiling point of water? ___________ 2) On the Fahrenhe ...
6. Absorption of Heat
... HRW 75E (5th ed.). Gas within a chamber passes through the cycle shown in Fig. 19-37. Determine the net heat added to the system during process CA if the heat QAB added during process AB is 20.0 J, no heat is transferred during process BC, and the net work dome during the cycle is 15.0 J. Since the ...
... HRW 75E (5th ed.). Gas within a chamber passes through the cycle shown in Fig. 19-37. Determine the net heat added to the system during process CA if the heat QAB added during process AB is 20.0 J, no heat is transferred during process BC, and the net work dome during the cycle is 15.0 J. Since the ...
Document
... distribution and heat transfer in one dimensional heat conduction problems associated with, large plane wall, a long cylinder, a sphere and a semi infinite medium. • Using a superposition approach call product solution, these charts can also be used to construct solutions for two dimensional transie ...
... distribution and heat transfer in one dimensional heat conduction problems associated with, large plane wall, a long cylinder, a sphere and a semi infinite medium. • Using a superposition approach call product solution, these charts can also be used to construct solutions for two dimensional transie ...
HeatTransfer
... Heat moves from higher temperature (higher kinetic energy) particles to lower temperature particles (lower kinetic energy.) e.g.: a cold spoon warms when placed in a cup of hot coffee. Thermal conductors transfer heat easily, while insulators do not. • Convection is the transfer of heat in flu ...
... Heat moves from higher temperature (higher kinetic energy) particles to lower temperature particles (lower kinetic energy.) e.g.: a cold spoon warms when placed in a cup of hot coffee. Thermal conductors transfer heat easily, while insulators do not. • Convection is the transfer of heat in flu ...
Heat Transfer - cloudfront.net
... • Explanation: Sun is heating up the ground more quickly than it heats the air, especially if the surface of the ground is a dark color. The heated air rises and bends light waves as it passes through them. Making the objects on the other side shimmer. ...
... • Explanation: Sun is heating up the ground more quickly than it heats the air, especially if the surface of the ground is a dark color. The heated air rises and bends light waves as it passes through them. Making the objects on the other side shimmer. ...
BCJ0205-15 Thermal phenomena (3-1-4)
... After taking this course the student should have acquired knowledge, intuition and mathematical skills in physical situations involving: ...
... After taking this course the student should have acquired knowledge, intuition and mathematical skills in physical situations involving: ...
Heat Calculations with Specific Heat
... • EX. How much heat is necessary to totally melt 5 g of ice at 0 C to liquid water at 0 C? • EX. How much heat is necessary to change 5 g of water at 100 C to steam at 100 C? ...
... • EX. How much heat is necessary to totally melt 5 g of ice at 0 C to liquid water at 0 C? • EX. How much heat is necessary to change 5 g of water at 100 C to steam at 100 C? ...