New Title - cloudfront.net
... related. As you read the section, compare and contrast temperature, thermal energy, and heat by completing the graphic organizer. The completed graphic organizer can be used to see the ways in which temperature, thermal energy, and heat are similar and different. Energy Measured ...
... related. As you read the section, compare and contrast temperature, thermal energy, and heat by completing the graphic organizer. The completed graphic organizer can be used to see the ways in which temperature, thermal energy, and heat are similar and different. Energy Measured ...
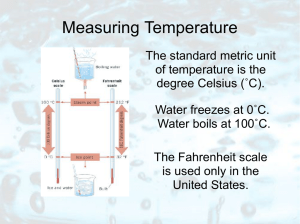
Measuring Temperature
... Heat depends on temperature, but also on the mass of the object, and its heat capacity. Even though Lake Ontario is at a colder temperature than your cup of coffee, it contains a lot more heat. The reason is that Lake Ontario is so much bigger (more massive) than your morning beverage. ...
... Heat depends on temperature, but also on the mass of the object, and its heat capacity. Even though Lake Ontario is at a colder temperature than your cup of coffee, it contains a lot more heat. The reason is that Lake Ontario is so much bigger (more massive) than your morning beverage. ...
Ch 16 Thermal Energy and Heat
... o The second law of thermodynamics stats that thermal energy can flow form colder objects to hotter objects only if works is done on the system. o Heat engine is any device that converts heat into work. o The efficiency of a heat engine is always less than 100 % o Waste heat is thermal energy that i ...
... o The second law of thermodynamics stats that thermal energy can flow form colder objects to hotter objects only if works is done on the system. o Heat engine is any device that converts heat into work. o The efficiency of a heat engine is always less than 100 % o Waste heat is thermal energy that i ...
Vocabulary - cloudfront.net
... Calculate the Ideal efficiency of a heat engine that takes in energy at 450 K and expels it to a reservoir at 60 K. ...
... Calculate the Ideal efficiency of a heat engine that takes in energy at 450 K and expels it to a reservoir at 60 K. ...
Heat Transfer conduction
... Heat energy is transferred from a high heat “source” to a low heat “sink”. Heat energy will “flow” from high temperature areas to low temperature ones through one of three methods; radiation, convection or conduction. Radiation is a mode of energy transfer that does not require a medium, or substanc ...
... Heat energy is transferred from a high heat “source” to a low heat “sink”. Heat energy will “flow” from high temperature areas to low temperature ones through one of three methods; radiation, convection or conduction. Radiation is a mode of energy transfer that does not require a medium, or substanc ...
Lecture 4: Heat transfer
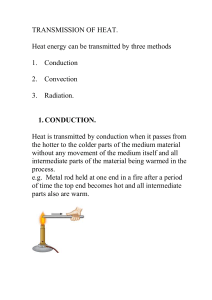
... 1. CONDUCTION. Heat is transmitted by conduction when it passes from the hotter to the colder parts of the medium material without any movement of the medium itself and all intermediate parts of the material being warmed in the process. e.g. Metal rod held at one end in a fire after a period of time ...
... 1. CONDUCTION. Heat is transmitted by conduction when it passes from the hotter to the colder parts of the medium material without any movement of the medium itself and all intermediate parts of the material being warmed in the process. e.g. Metal rod held at one end in a fire after a period of time ...
Heat and Thermal Energy Word Problems
... from 20.0oC to 30.0oC. Calculate the specific heat of the metal. 14. A copper wire has a mass of 165 g. An electric current runs through the wire for a short time and its temperature rises from 21oC to 39oC. What minimum quantity of energy is converted by the electric current? 15. A 100 g mass of tu ...
... from 20.0oC to 30.0oC. Calculate the specific heat of the metal. 14. A copper wire has a mass of 165 g. An electric current runs through the wire for a short time and its temperature rises from 21oC to 39oC. What minimum quantity of energy is converted by the electric current? 15. A 100 g mass of tu ...
Thermochemistry
... Conservation of Energy ◦ Energy is not created or destroyed in a physical or chemical process ◦ If energy in a system decreases, then the energy of the surroundings increases by the same amount ...
... Conservation of Energy ◦ Energy is not created or destroyed in a physical or chemical process ◦ If energy in a system decreases, then the energy of the surroundings increases by the same amount ...
WORKSHOP 2: Dimensional Analysis and
... 7. A rubber stopper weighing 65.4 g is immersed into a graduated cylinder filled with 30.0 mL of liquid. The liquid level then rises to 48.8 mL. Calculate the density of the stopper. 3.48 g/mL 8. If the density of a liquid is known to be 0.785 g/mL, calculate the mass of the liquid if its volume is ...
... 7. A rubber stopper weighing 65.4 g is immersed into a graduated cylinder filled with 30.0 mL of liquid. The liquid level then rises to 48.8 mL. Calculate the density of the stopper. 3.48 g/mL 8. If the density of a liquid is known to be 0.785 g/mL, calculate the mass of the liquid if its volume is ...
Thermal Energy - Cloudfront.net
... made of particles and atoms that constantly and randomly move. All atoms are in constant, random motion, ALL the time---even in your body! The faster they move, the more KE they have. Hot object’s particles move faster than an object that is cold. ...
... made of particles and atoms that constantly and randomly move. All atoms are in constant, random motion, ALL the time---even in your body! The faster they move, the more KE they have. Hot object’s particles move faster than an object that is cold. ...
Conceptual Physics. Tenth Edition
... from being cold, but this will require another essay… Second, you don’t want your coat to be warm, although that feels comfortable when you put it on, but you want your coat to keep you warm! How does this work according to the physics of heat transfer? According to the second law of thermodynamics ...
... from being cold, but this will require another essay… Second, you don’t want your coat to be warm, although that feels comfortable when you put it on, but you want your coat to keep you warm! How does this work according to the physics of heat transfer? According to the second law of thermodynamics ...
2, 5, 9, 11, 18, 20 / 3, 9, 10, 16, 19, 24
... sandwiched together to form a layered slab. The two pieces have the same thickness and cross-sectional area. The exposed surfaces have constant temperatures. The temperature of the exposed Styrofoam surface is greater than the temperature of the exposed wood surface. The rate of heat flow through ei ...
... sandwiched together to form a layered slab. The two pieces have the same thickness and cross-sectional area. The exposed surfaces have constant temperatures. The temperature of the exposed Styrofoam surface is greater than the temperature of the exposed wood surface. The rate of heat flow through ei ...
241 Lecture 11
... Equilibrium In thermodynamics, equilibrium means not only the absence of change but the absence of any tendency toward change on a macroscopic scale. Different kinds of driving forces bring about different kinds of change. For example: imbalance of mechanical forces tend to cause energy ...
... Equilibrium In thermodynamics, equilibrium means not only the absence of change but the absence of any tendency toward change on a macroscopic scale. Different kinds of driving forces bring about different kinds of change. For example: imbalance of mechanical forces tend to cause energy ...
Note: Moving air
... Answer: The shiny surface of foil reflects infrared radiation, which would reduce heating from outside radiation. The surface also does not emit IR very well, so it would lose less energy by radiation than a non-shiny surface. ...
... Answer: The shiny surface of foil reflects infrared radiation, which would reduce heating from outside radiation. The surface also does not emit IR very well, so it would lose less energy by radiation than a non-shiny surface. ...
Geology :: 3. Energy and the Dynamic Earth
... One of the fundamental laws of nature is that heat always flows from a hot place to cold one. Thus, heat must flow outward from the interior of the Earth toward the cool surface. The process by which heat can move through solid rock, or any other solid body, without deforming the solid is called con ...
... One of the fundamental laws of nature is that heat always flows from a hot place to cold one. Thus, heat must flow outward from the interior of the Earth toward the cool surface. The process by which heat can move through solid rock, or any other solid body, without deforming the solid is called con ...
Consider a rigid tank with a movable piston
... Air-Standard Assumptions: 1) The working fluid is air, which continuously circulates in a closed loop and always behaves as an ideal gas. 2) All the processes that make up the cycle are internally reversible. 3) The combustion process is replaced by a heat-addition process from an external source. 4 ...
... Air-Standard Assumptions: 1) The working fluid is air, which continuously circulates in a closed loop and always behaves as an ideal gas. 2) All the processes that make up the cycle are internally reversible. 3) The combustion process is replaced by a heat-addition process from an external source. 4 ...
Ch3_HeatTransfer_5
... ratio of the temperature difference, dT, to the heat transfer Q. This is analogous to Ohm's law, in which the electrical resistance is defined as the ratio of the voltage drop across a resistor to the current flow across the resistor. ...
... ratio of the temperature difference, dT, to the heat transfer Q. This is analogous to Ohm's law, in which the electrical resistance is defined as the ratio of the voltage drop across a resistor to the current flow across the resistor. ...
Chapter 19
... Fig. 19.2W, Callister 6e. (Fig. 19.2W adapted from L.J. Korb, C.A. Morant, R.M. Calland, and C.S. Thatcher, "The Shuttle Orbiter Thermal Protection System", Ceramic Bulletin, No. 11, Nov. 1981, p. 1189.) ...
... Fig. 19.2W, Callister 6e. (Fig. 19.2W adapted from L.J. Korb, C.A. Morant, R.M. Calland, and C.S. Thatcher, "The Shuttle Orbiter Thermal Protection System", Ceramic Bulletin, No. 11, Nov. 1981, p. 1189.) ...
Transfer of Thermal Energy worksheet
... Convection is the transfer of heat energy in a fluid. This type of heating is most commonly seen in the kitchen when you see liquid boiling. Air in the atmosphere acts as a fluid. The sun's radiation strikes the ground, thus warming the rocks. As the rock's temperature rises due to conduction, heat ...
... Convection is the transfer of heat energy in a fluid. This type of heating is most commonly seen in the kitchen when you see liquid boiling. Air in the atmosphere acts as a fluid. The sun's radiation strikes the ground, thus warming the rocks. As the rock's temperature rises due to conduction, heat ...