Pioneer Science Worksheet
... A. Temperature is the measure of the average energy of molecular motion in a substance. B. Temperature is also known as thermal energy. C. Temperature does not depend on the size or type ...
... A. Temperature is the measure of the average energy of molecular motion in a substance. B. Temperature is also known as thermal energy. C. Temperature does not depend on the size or type ...
Read-Around therm = heat, temperature
... names the device in a home or classroom that helps you adjust the temperature so that it is not too hot or too cold ? ...
... names the device in a home or classroom that helps you adjust the temperature so that it is not too hot or too cold ? ...
More Carnot Cycle March 4, 2010 Efficiency = W/Qin = Qin
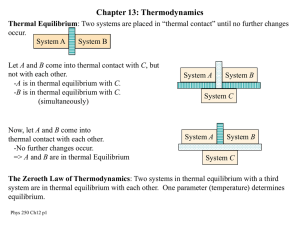
... The diagram shows a rod of a conducting material with cross sectional area A and length L. The left end of the rod is kept at constant temperature TH and the right end at lower temperature TC. The sides of the rod are perfectly translated so that there is no heat transfer to the environment. When a ...
... The diagram shows a rod of a conducting material with cross sectional area A and length L. The left end of the rod is kept at constant temperature TH and the right end at lower temperature TC. The sides of the rod are perfectly translated so that there is no heat transfer to the environment. When a ...
Chapter 5 PPT 2 - Kawameeh Middle School
... The outside of the tube gets heated The particles of the liquid (alcohol) inside speeds up and the liquid spreads out . The height of the alcohol indicates the temperature ...
... The outside of the tube gets heated The particles of the liquid (alcohol) inside speeds up and the liquid spreads out . The height of the alcohol indicates the temperature ...
Thermal Energy - St. Thomas the Apostle School
... Matter in motion • The matter around you is made of tiny particlesatoms and molecules • These particles are in constant motion • Because they are moving they have kinetic energy • The faster they move the more kinetic energy they have • These particles move faster in hot objects then cooler objects ...
... Matter in motion • The matter around you is made of tiny particlesatoms and molecules • These particles are in constant motion • Because they are moving they have kinetic energy • The faster they move the more kinetic energy they have • These particles move faster in hot objects then cooler objects ...
one dimensional steady state heat conduction
... One dimensional: If heat is flowing in only one coordinate direction, then it follows that there is no temperature gradient in the other two directions. Thus the two partials associated with these directions are equal to zero. Two dimensional: If heat is flowing in only two coordinate directions, th ...
... One dimensional: If heat is flowing in only one coordinate direction, then it follows that there is no temperature gradient in the other two directions. Thus the two partials associated with these directions are equal to zero. Two dimensional: If heat is flowing in only two coordinate directions, th ...
module 2
... One dimensional: If heat is flowing in only one coordinate direction, then it follows that there is no temperature gradient in the other two directions. Thus the two partials associated with these directions are equal to zero. Two dimensional: If heat is flowing in only two coordinate directions, th ...
... One dimensional: If heat is flowing in only one coordinate direction, then it follows that there is no temperature gradient in the other two directions. Thus the two partials associated with these directions are equal to zero. Two dimensional: If heat is flowing in only two coordinate directions, th ...
Buffet_geoneutrino - University of Hawaii Physics and Astronomy
... Addition of light elements required to explain density (popular suggestions include O, S, Si ) ...
... Addition of light elements required to explain density (popular suggestions include O, S, Si ) ...
Name
... Part A: Match the terms on the left with the explanations and situations on the right. Some answers may be used more than once and some may not be used at all. A. method of heat transfer where particles collide 1. Heat flow B. heat flows slowly in this type of material 2. Convection 3. Thermal Energ ...
... Part A: Match the terms on the left with the explanations and situations on the right. Some answers may be used more than once and some may not be used at all. A. method of heat transfer where particles collide 1. Heat flow B. heat flows slowly in this type of material 2. Convection 3. Thermal Energ ...
Thermodynamic Efficiency Energy Conversion Efficiency Ideal Gas
... but what happens at 4000 degrees? ...
... but what happens at 4000 degrees? ...
how to wire electric heat relays - Grover Electric and Plumbing Supply
... situation, but our experience has been that they do not perform to the standards most people desire. Another attractive benefit derived from using this method of heat control is less variation between high and low room temperatures, thus a possible savings in energy use and therefore money. Most lin ...
... situation, but our experience has been that they do not perform to the standards most people desire. Another attractive benefit derived from using this method of heat control is less variation between high and low room temperatures, thus a possible savings in energy use and therefore money. Most lin ...
Midterm Examination
... (a) If 11.0 mol of an ideal gas is put into the tank at a temperature of 23.00C, to what temperature can the gas be warmed before the tank ruptures? You can ignore the thermal expansion of the tank. (b) Based on your answer to part (a), is it reasonable to ignore the thermal expansion of the tank? E ...
... (a) If 11.0 mol of an ideal gas is put into the tank at a temperature of 23.00C, to what temperature can the gas be warmed before the tank ruptures? You can ignore the thermal expansion of the tank. (b) Based on your answer to part (a), is it reasonable to ignore the thermal expansion of the tank? E ...
Test Review-Atmosphere Intro
... Test 2: Intro/Properties of Earth’s Atmosphere The following is a list of topics to help guide you in your studies. This is not to be used as your only source of studying!!! Topics on the exam may include but are not limited to the following: 1. ESRT Temperature & Pressure a. Reading both charts, co ...
... Test 2: Intro/Properties of Earth’s Atmosphere The following is a list of topics to help guide you in your studies. This is not to be used as your only source of studying!!! Topics on the exam may include but are not limited to the following: 1. ESRT Temperature & Pressure a. Reading both charts, co ...