Chapter 2 Boiler 101: typical NYC residential heating system
... For a natural gas-fired hot water boiler, almost all of the components are the same except for the equipment used to supply fuel to the burner (the gas train). A natural gasfired heater does not include a fuel pump because the natural gas fuel is not a liquid. Instead it includes a connection to the ...
... For a natural gas-fired hot water boiler, almost all of the components are the same except for the equipment used to supply fuel to the burner (the gas train). A natural gasfired heater does not include a fuel pump because the natural gas fuel is not a liquid. Instead it includes a connection to the ...
States of Matter
... Variables are pressure (y) and temperature (x) Triple point: temperature and pressure where solid, liquid and gas can exist in equilibrium Critical temperature: temperature above which a substance cannot be liquified Critical pressure: pressure necessary to liquefy a substance at the critical temper ...
... Variables are pressure (y) and temperature (x) Triple point: temperature and pressure where solid, liquid and gas can exist in equilibrium Critical temperature: temperature above which a substance cannot be liquified Critical pressure: pressure necessary to liquefy a substance at the critical temper ...
heat
... determine the enthalpy change associated with other processes, such as: Chemical reactions* (bond energies) Phase changes* (intermolecular forces) Mixing (intermolecular forces) Solvation (intermolecular forces) *These are the processes you will be learning today. ...
... determine the enthalpy change associated with other processes, such as: Chemical reactions* (bond energies) Phase changes* (intermolecular forces) Mixing (intermolecular forces) Solvation (intermolecular forces) *These are the processes you will be learning today. ...
Exercises - Madison County Schools
... 32. In order to quantify heat, we must specify the and of substance affected. 33. Suppose you place a pot with 1 cup of water and an identical pot with 2 cups of water on a hot stove for the same amount of time. Circle the letters beside the sentences that correctly describe what happens. a. More he ...
... 32. In order to quantify heat, we must specify the and of substance affected. 33. Suppose you place a pot with 1 cup of water and an identical pot with 2 cups of water on a hot stove for the same amount of time. Circle the letters beside the sentences that correctly describe what happens. a. More he ...
Physics Semester 1 Review
... 6. What is the minimum amount of energy required to completely melt a 5-kg lead brick which has a starting temperature of 20 °C? The melting point of lead is 328 °C. The specific heat capacity of lead is 128 J/(kg·C°); and its latent heat of fusion is 23200 J/kg. 7. A blue supergiant star has a radi ...
... 6. What is the minimum amount of energy required to completely melt a 5-kg lead brick which has a starting temperature of 20 °C? The melting point of lead is 328 °C. The specific heat capacity of lead is 128 J/(kg·C°); and its latent heat of fusion is 23200 J/kg. 7. A blue supergiant star has a radi ...
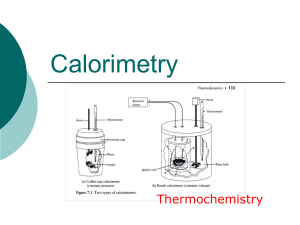
Calorimetry
... efficient. Heat radiates in all directions, so not all of it was absorbed by the calorimeter. Energy may have also been lost through other mechanisms, such as sound. In addition, incomplete chemical combustion may have occurred which would carry unburned portions of the peanut away in the form of s ...
... efficient. Heat radiates in all directions, so not all of it was absorbed by the calorimeter. Energy may have also been lost through other mechanisms, such as sound. In addition, incomplete chemical combustion may have occurred which would carry unburned portions of the peanut away in the form of s ...
calorimetry - Saddleback College
... The textbook discusses the transformation of energy from one form to another; e.g. from mechanical energy to thermal energy, from chemical energy to thermal energy to mechanical energy, and so on. In this experiment you will investigate some of the properties of thermal energy in transit or heat. Yo ...
... The textbook discusses the transformation of energy from one form to another; e.g. from mechanical energy to thermal energy, from chemical energy to thermal energy to mechanical energy, and so on. In this experiment you will investigate some of the properties of thermal energy in transit or heat. Yo ...
The laws of thermodynamics
... At constant pressure, the change in enthalpy is equal to the heat The change of enthalpy is independent of path. Q: Does q or W depend on path? For the change involving solids and liquids, HU, but for gases, HU Q:explain why? ...
... At constant pressure, the change in enthalpy is equal to the heat The change of enthalpy is independent of path. Q: Does q or W depend on path? For the change involving solids and liquids, HU, but for gases, HU Q:explain why? ...
MAE 320 – Thermodynamics
... the mechanical work which, in turn, is converted to electricity by the producer. Obviously, the engine efficiency is defined as the ratio of the mechanical power to the consumption rate chemical energy; while the producer efficiency is defined as the ratio of the output of electrical power to mechan ...
... the mechanical work which, in turn, is converted to electricity by the producer. Obviously, the engine efficiency is defined as the ratio of the mechanical power to the consumption rate chemical energy; while the producer efficiency is defined as the ratio of the output of electrical power to mechan ...
Practice Exam 3 - University of Missouri
... H2O(g): –241.8 kJ/mol, what is the enthalpy change for the combustion of a mol of n-hexanol, to form gaseous CO2 and H2O? a. 3984 kJ b. –3676.1 kJ c. –3984 kJ d. +3676.1 kJ ...
... H2O(g): –241.8 kJ/mol, what is the enthalpy change for the combustion of a mol of n-hexanol, to form gaseous CO2 and H2O? a. 3984 kJ b. –3676.1 kJ c. –3984 kJ d. +3676.1 kJ ...
Done by: Terence Lee (27) - ScienceIMPORTANTRCYJTLCEC
... between them. As conduction (as stated above) transfers by physical contact due to the fact that particles transfer kinetic energy by interacting with neighbor particles, we decided to leave a gap so as to prevent conduction of heat from the surrounding to the ice cube. The decrease in conduction of ...
... between them. As conduction (as stated above) transfers by physical contact due to the fact that particles transfer kinetic energy by interacting with neighbor particles, we decided to leave a gap so as to prevent conduction of heat from the surrounding to the ice cube. The decrease in conduction of ...
The Geosphere
... Heat transfer through the motion of a hot material When a material heats, it expands The expanded material is less dense The material then rises, carrying heat ...
... Heat transfer through the motion of a hot material When a material heats, it expands The expanded material is less dense The material then rises, carrying heat ...
thermochem-prob-solns
... (20g)(4.184 J/g-K)( 90C-Teq) = (30g)(4.184 J/g-K)(Teq - 6.0C) 7531.2 - 83.68Teq = 125.52Teq - 753.12 209.2Teq = 8284.32 Teq = 39.6C ; Teq is always between warm and cool temperature. Bomb Calorimerty- For this condition, q = Cpbomb x T ; Cpbomb is heat capacity of the bomb calorimeter. Ex. 4.00 ...
... (20g)(4.184 J/g-K)( 90C-Teq) = (30g)(4.184 J/g-K)(Teq - 6.0C) 7531.2 - 83.68Teq = 125.52Teq - 753.12 209.2Teq = 8284.32 Teq = 39.6C ; Teq is always between warm and cool temperature. Bomb Calorimerty- For this condition, q = Cpbomb x T ; Cpbomb is heat capacity of the bomb calorimeter. Ex. 4.00 ...
PPT Slide Show
... • Heat transfer explains the transfer of thermal energy, between physical systems depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation. ...
... • Heat transfer explains the transfer of thermal energy, between physical systems depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation. ...
Energy Savings Through Radiant Heat
... your heating bill is a winning combination. Multiple zoning, thermal mass, off-peak rates, even heat distribution and lower temperature settings are just some of the strategies that reduce energy bills with radiant heating. Multiple zoning allows you to turn down thermostats in rooms not be used. Ev ...
... your heating bill is a winning combination. Multiple zoning, thermal mass, off-peak rates, even heat distribution and lower temperature settings are just some of the strategies that reduce energy bills with radiant heating. Multiple zoning allows you to turn down thermostats in rooms not be used. Ev ...
Lesson 3-2 - TeacherWeb
... partly on temperature. Something at a high temperature has more thermal energy than something at a low temperature. ...
... partly on temperature. Something at a high temperature has more thermal energy than something at a low temperature. ...
Heat and Energy
... (J), depends partly on temperature. Something at a high temperature has more thermal energy than something at a low temperature. ...
... (J), depends partly on temperature. Something at a high temperature has more thermal energy than something at a low temperature. ...
Thermochemistry - hrsbstaff.ednet.ns.ca
... Note: When a styrene (Styrofoam) cup is used, often it is ignored in the calculations. It is assumed that the heat absorbed by the cup is negligible. If the heat absorbed by the cup should be included in the calculations, information about the heat capacity or specific heat capacity will be given in ...
... Note: When a styrene (Styrofoam) cup is used, often it is ignored in the calculations. It is assumed that the heat absorbed by the cup is negligible. If the heat absorbed by the cup should be included in the calculations, information about the heat capacity or specific heat capacity will be given in ...
Exercise 1-2 - MyCourses
... 1. a) Derive an equation which describes cooling of an object which is a good thermal conductor. Assume uniform heat transfer outside the surface of the object. b) Spherical iron ball with diameter of 1 cm is initially at 100 oC temperature. Air at temperature 20 oC flows outside it. Calculate tempe ...
... 1. a) Derive an equation which describes cooling of an object which is a good thermal conductor. Assume uniform heat transfer outside the surface of the object. b) Spherical iron ball with diameter of 1 cm is initially at 100 oC temperature. Air at temperature 20 oC flows outside it. Calculate tempe ...
Physical Science Day 2 - Fall River Public Schools
... Melting Point: a unique property of every metal, a way to identify metals. It is the point at which a solid turns into a liquid. Boiling Point: a unique property of every liquid, a way to identify liquids. It is the point at which a liquid turns into a gas. Physical Change: change the shape or struc ...
... Melting Point: a unique property of every metal, a way to identify metals. It is the point at which a solid turns into a liquid. Boiling Point: a unique property of every liquid, a way to identify liquids. It is the point at which a liquid turns into a gas. Physical Change: change the shape or struc ...
Final Exam Spring 2001 Phy 231 Form 1
... the right choice. For example: if you get 4.432156 and one of the choices given is 4.4, then the later is the answer. Similarly, if you get 5.6772 and one of the choices is 5.68, then it should be considered as the right choice. ----------------------------------------------------------------------- ...
... the right choice. For example: if you get 4.432156 and one of the choices given is 4.4, then the later is the answer. Similarly, if you get 5.6772 and one of the choices is 5.68, then it should be considered as the right choice. ----------------------------------------------------------------------- ...
CH3080_reportsample_formaterrors
... is returned by capillary action. In summary: inside a heat pipe, "hot" vapor flows in one direction, condenses to the liquid phase, which flows back in the other direction to evaporate again and close the cycle. One reason for the effectiveness of heat pipes is the amount of heat that an evaporating ...
... is returned by capillary action. In summary: inside a heat pipe, "hot" vapor flows in one direction, condenses to the liquid phase, which flows back in the other direction to evaporate again and close the cycle. One reason for the effectiveness of heat pipes is the amount of heat that an evaporating ...