specific heat
... How much energy would be needed to heat 450 g of copper metal from 25.0 ºC to 75.0 ºC? The specific heat of copper at 25.0 ºC is 0.385 J/g ºC. ...
... How much energy would be needed to heat 450 g of copper metal from 25.0 ºC to 75.0 ºC? The specific heat of copper at 25.0 ºC is 0.385 J/g ºC. ...
Phases of Matter - Bill Nye the Science Guy Wkst
... 14. To change a solid to a liquid, you need to increase / decrease the amount of heat energy . ...
... 14. To change a solid to a liquid, you need to increase / decrease the amount of heat energy . ...
Worksheet 33
... PHYS 221 SI Worksheet 1. A gas in a cylinder is held at a constant pressure of 2.30E5 Pa and is cooled and compressed from 1.70 m^3 to 1.20 m^3. The internal energy of the gas decreases by 1.40E5 J. (a) Find the work done by the gas. (b) Find the absolute value |Q| of the heat flow into or out of th ...
... PHYS 221 SI Worksheet 1. A gas in a cylinder is held at a constant pressure of 2.30E5 Pa and is cooled and compressed from 1.70 m^3 to 1.20 m^3. The internal energy of the gas decreases by 1.40E5 J. (a) Find the work done by the gas. (b) Find the absolute value |Q| of the heat flow into or out of th ...
Heat Transfer Comparison in Coaxial Tube in Tube Heat Exchanger
... are currently used in R&AC systems, in accordance with the Montreal Protocol, actual is exchange of those by new, ecologically acceptable, HFC refrigerants. Therefore system performance analyses was made where the single component refrigerant R22 was replaced with zeotropic mixture R407C. In the sys ...
... are currently used in R&AC systems, in accordance with the Montreal Protocol, actual is exchange of those by new, ecologically acceptable, HFC refrigerants. Therefore system performance analyses was made where the single component refrigerant R22 was replaced with zeotropic mixture R407C. In the sys ...
Types of Heat Transfer
... • Takes place in fluids such as liquids or air. • Occurs when hot air or liquid rises and cold air or liquid moves in to take its place. ...
... • Takes place in fluids such as liquids or air. • Occurs when hot air or liquid rises and cold air or liquid moves in to take its place. ...
14_Water Cooling System
... 1. HEAT SOURCES Burning of fuel Heat developed by compression of air Frictional heat ...
... 1. HEAT SOURCES Burning of fuel Heat developed by compression of air Frictional heat ...
Note: Moving air
... Question: Describe how conduction, convection, and radiation play a role in losing heat through a double-pane window. Answer: Heat is conducted through the solid glass and through the four air films, two on each side of each sheet of glass. The air films do the bulk of the insulating! If the space b ...
... Question: Describe how conduction, convection, and radiation play a role in losing heat through a double-pane window. Answer: Heat is conducted through the solid glass and through the four air films, two on each side of each sheet of glass. The air films do the bulk of the insulating! If the space b ...
Temperature Conversions
... 4. A 400g glass coffee cup is at room temperature, 20.0ºC. It is then plunged into hot dishwater, 80.0ºC. If the temperature of the cup reaches that of the dishwater, how much heat does the cup absorb? Assume the mass of the dishwater is large enough so its temperature doesn’t change appreciably. ...
... 4. A 400g glass coffee cup is at room temperature, 20.0ºC. It is then plunged into hot dishwater, 80.0ºC. If the temperature of the cup reaches that of the dishwater, how much heat does the cup absorb? Assume the mass of the dishwater is large enough so its temperature doesn’t change appreciably. ...
File
... A 9.84 oz ingot of unknown metal is heated from 73.2 °F to 191.2 °F. This requires 3.91 kcal of energy. Calculate the specific heat of the metal and determine its identity. ...
... A 9.84 oz ingot of unknown metal is heated from 73.2 °F to 191.2 °F. This requires 3.91 kcal of energy. Calculate the specific heat of the metal and determine its identity. ...
Thermal Energy
... lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
... lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
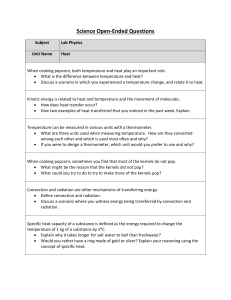
Physics-Heat OEQs
... Specific heat capacity of a substance is defined as the energy required to change the temperature of 1 kg of a substance by 1⁰C. Explain why it takes longer for salt water to boil than freshwater? Would you rather have a ring made of gold or silver? Explain your reasoning using the concept of sp ...
... Specific heat capacity of a substance is defined as the energy required to change the temperature of 1 kg of a substance by 1⁰C. Explain why it takes longer for salt water to boil than freshwater? Would you rather have a ring made of gold or silver? Explain your reasoning using the concept of sp ...
Specific Heat Capacity - Tasker Milward
... Specific Heat Capacity • The specific heat capacity is the amount of energy required to increase the temperature of 1kg of a substance by 1˚C • We will calculate the specific heat capacity of water by heating it with an electrical heater and measuring the energy required for a fixed temperature ris ...
... Specific Heat Capacity • The specific heat capacity is the amount of energy required to increase the temperature of 1kg of a substance by 1˚C • We will calculate the specific heat capacity of water by heating it with an electrical heater and measuring the energy required for a fixed temperature ris ...
Chapters 1 and 2
... Temperature is a measure of the tendency of an object to spontaneously give up/absorb energy to/from its surroundings. Diathermal wall is a system boundary that freely allows heat to be exchanged, i.e. very short relaxation time for systems separated by a diathermal wall. Adiabatic wall is a system ...
... Temperature is a measure of the tendency of an object to spontaneously give up/absorb energy to/from its surroundings. Diathermal wall is a system boundary that freely allows heat to be exchanged, i.e. very short relaxation time for systems separated by a diathermal wall. Adiabatic wall is a system ...
problem-set-7c-cal-2016
... The specific heat of silver is 0.24 J/g . Co . How much heat needs to be added to a 184.2 g block of silver to get its temperature to rise 12.7 degrees? ...
... The specific heat of silver is 0.24 J/g . Co . How much heat needs to be added to a 184.2 g block of silver to get its temperature to rise 12.7 degrees? ...
Glossary of Terms - NJR Home Services
... By the same token, a 100,000 Btuh furnace that is 90% efficient only sends 10% of the total heat out the chimney - thus burning less gas to get the same results and reducing your gas heating costs. Ton - You'll often see this as a measurement of the capacity of an air conditioning system. Don't pan ...
... By the same token, a 100,000 Btuh furnace that is 90% efficient only sends 10% of the total heat out the chimney - thus burning less gas to get the same results and reducing your gas heating costs. Ton - You'll often see this as a measurement of the capacity of an air conditioning system. Don't pan ...
Calorimetry: Heat of Fusion of Ice Procedure In a 250 mL beaker
... Immediately add 2-3 ice cubes. Stir the mixture carefully. The cup should contain ice at all times. If the last of the ice is about to melt, add another ice cube. Monitor the temperature of the mixture as you stir. Continue stirring until the temperature no longer drops. Record this final temperatur ...
... Immediately add 2-3 ice cubes. Stir the mixture carefully. The cup should contain ice at all times. If the last of the ice is about to melt, add another ice cube. Monitor the temperature of the mixture as you stir. Continue stirring until the temperature no longer drops. Record this final temperatur ...
Physics 41 Exam 3 Practice HW
... 5. A bridge is made with segments of concrete 50 m long. If the linear expansion coefficient is 12 10–6 / °C how much spacing (in cm) is needed to allow for expansion during an extreme temperature change of 150°F? a. 10 b. 2.5 c. 7.5 d. 5.0 e. 9.5 ...
... 5. A bridge is made with segments of concrete 50 m long. If the linear expansion coefficient is 12 10–6 / °C how much spacing (in cm) is needed to allow for expansion during an extreme temperature change of 150°F? a. 10 b. 2.5 c. 7.5 d. 5.0 e. 9.5 ...
First Law of Thermodynamics
... Boiling •Is not a temperature •It is a pressure •Pressure above the liquid=pressure from particles leaving the surface ...
... Boiling •Is not a temperature •It is a pressure •Pressure above the liquid=pressure from particles leaving the surface ...
Entropy - Dordt College Homepages
... atomic collisions are time-reversal invariant (S 0 doesn’t apply) ball bouncing - forward direction is one where successive bounces get smaller S > 0 same as t > 0 (tautology) no ...
... atomic collisions are time-reversal invariant (S 0 doesn’t apply) ball bouncing - forward direction is one where successive bounces get smaller S > 0 same as t > 0 (tautology) no ...
Problem #1 Water is boiled at Tsat = 100°C by a spherical platinum
... in the figure. The tubes have an outer diameter of 3.8cm and inner diameter of 3cm. The flow is such that the internal convection coefficient is 4,000 W/m2.°C and the tubes are made of a material with conductivity of k = 401 W/m.°C. The average value of overall heat transfer coefficient and the tube ...
... in the figure. The tubes have an outer diameter of 3.8cm and inner diameter of 3cm. The flow is such that the internal convection coefficient is 4,000 W/m2.°C and the tubes are made of a material with conductivity of k = 401 W/m.°C. The average value of overall heat transfer coefficient and the tube ...
Understanding Heat Transfers Conduction, Convection and Radiation
... •heat up to room temperature. ...
... •heat up to room temperature. ...
saving with heat pumps
... your zone—zones 1 and 2 (map below) are generally warm enough to allow heat pumps to operate efficiently. Generally, the yearly average ambient temperature should be 19 degrees Celsius or higher how many STCs—the number of STCs (or Small-scale Technology Certificates) for each system also depends on ...
... your zone—zones 1 and 2 (map below) are generally warm enough to allow heat pumps to operate efficiently. Generally, the yearly average ambient temperature should be 19 degrees Celsius or higher how many STCs—the number of STCs (or Small-scale Technology Certificates) for each system also depends on ...
thermodynamics, heat and mass transfer
... THERMODYNAMICS, HEAT AND MASS TRANSFER TUTORIAL NO: 3 ...
... THERMODYNAMICS, HEAT AND MASS TRANSFER TUTORIAL NO: 3 ...