Cell Respiration II: Fermentation reactions
... Cell Respiration II: Fermentation reactions During strenuous exercise or when O2 is unavailable or low in the environment, ATP production still needs to occur for life to continue. Fermentation reactions allow ATP to be produced and pyruvate (or its derivative) becomes the terminal electron acceptor ...
... Cell Respiration II: Fermentation reactions During strenuous exercise or when O2 is unavailable or low in the environment, ATP production still needs to occur for life to continue. Fermentation reactions allow ATP to be produced and pyruvate (or its derivative) becomes the terminal electron acceptor ...
logcsscibap_2_4_2_c_..
... Are the shapes of the reactant and active site similar or different? Explain your answer. (2 marks) ...
... Are the shapes of the reactant and active site similar or different? Explain your answer. (2 marks) ...
USED Enzymes Worksheet
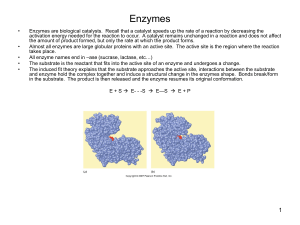
... Because enzymes are made in living things they are called ____________________ (or organic catalysts) Proteins are made when ____________________ join together. The function of a protein is decided not only by the _______________ of amino acids but also by the way the protein __________. Most enzyme ...
... Because enzymes are made in living things they are called ____________________ (or organic catalysts) Proteins are made when ____________________ join together. The function of a protein is decided not only by the _______________ of amino acids but also by the way the protein __________. Most enzyme ...
Nutrition and addiction – can dietary changes assist with recovery? [3]
... are not recognised by enzymes and may end up stored in fat cells or, worse, they may interfere with biochemical processes. On the other hand, fresh food, or food properly prepared and cooked, retains macro- and micronutrients, fibres and water that are essential for correct functioning of the body a ...
... are not recognised by enzymes and may end up stored in fat cells or, worse, they may interfere with biochemical processes. On the other hand, fresh food, or food properly prepared and cooked, retains macro- and micronutrients, fibres and water that are essential for correct functioning of the body a ...
History of enzymology
... 1900) first used the term enzyme , which comes from Greek ενζυμον (enzymon)"in leaven", to describe this process. The word enzyme was used later to refer to nonliving substances such as pepsin, and the word ferment used to refer to chemical activity produced by living organisms. In 1897 Eduard Buchn ...
... 1900) first used the term enzyme , which comes from Greek ενζυμον (enzymon)"in leaven", to describe this process. The word enzyme was used later to refer to nonliving substances such as pepsin, and the word ferment used to refer to chemical activity produced by living organisms. In 1897 Eduard Buchn ...
What are the functions of the Liver?
... How are fatty acids (from fats) utilized? • least to be broken down • converted by the liver, to forms that can be oxidised and stored • used to build protoplasm (e.g. in cell membrane) ...
... How are fatty acids (from fats) utilized? • least to be broken down • converted by the liver, to forms that can be oxidised and stored • used to build protoplasm (e.g. in cell membrane) ...
Enzymes - Cedar City
... molecules, thus will not be capable to utilize the FOG as a food source. If this occurs the eventual accumulation of fatty acid, volatile organic acid and others, may accumulate and thus lower the pH within the interceptor body to a point where the pH is at or below 4.0. Additionally, the importance ...
... molecules, thus will not be capable to utilize the FOG as a food source. If this occurs the eventual accumulation of fatty acid, volatile organic acid and others, may accumulate and thus lower the pH within the interceptor body to a point where the pH is at or below 4.0. Additionally, the importance ...
Chapter 4 Enzymes and Energy
... • Different organs may make different enzymes (isoenzymes) that have the same activity. – Differences in structure do not affect the active sites. ...
... • Different organs may make different enzymes (isoenzymes) that have the same activity. – Differences in structure do not affect the active sites. ...
Enzymes - CynthiaJankowski
... reactants of the reaction that it is catalyzing. • The reactant an enzyme works on is called a substrate. • The substrate binds to the active site to make the enzyme active. • Factors such as temperature, and pH affect enzyme activity. ...
... reactants of the reaction that it is catalyzing. • The reactant an enzyme works on is called a substrate. • The substrate binds to the active site to make the enzyme active. • Factors such as temperature, and pH affect enzyme activity. ...
Enzyme Quiz # 20 First : Last: 1. Explain how an enzyme speeds up
... 2. Explain how the body is able to activate an enzyme in one part of the digestive tract (i.e stomach) and then denature the enzyme in later parts of the digestive tract ( i.e small intestine ) and at the same time activate other digestive enzymes in the small intestine to help this organ fulfill it ...
... 2. Explain how the body is able to activate an enzyme in one part of the digestive tract (i.e stomach) and then denature the enzyme in later parts of the digestive tract ( i.e small intestine ) and at the same time activate other digestive enzymes in the small intestine to help this organ fulfill it ...
A1983QQ90800002
... Rhodopseudomonas spheroides to obtain a partially purified preparation of the enzyme and to show that it could indeed be used for the enzymic determination of acetoacetate and hydroxybutyrate. Perhaps more importantly, the enzyme appeared stable. It was at this stage that Jane Mellanby, a PhD studen ...
... Rhodopseudomonas spheroides to obtain a partially purified preparation of the enzyme and to show that it could indeed be used for the enzymic determination of acetoacetate and hydroxybutyrate. Perhaps more importantly, the enzyme appeared stable. It was at this stage that Jane Mellanby, a PhD studen ...
ToothpickasePreLab
... chart should be detailed enough that an elementary school student can follow them and complete the lab. ...
... chart should be detailed enough that an elementary school student can follow them and complete the lab. ...
Comparison of the chaperone-like activity of camel and bovine β
... and structural zinc from the enzyme leading to whole loss of functionality. Diverse forms of YADH (native, apo-I and apo-II) are differed in surface hydrophobicity; thereby, exhibiting different interactions with a chaperone molecule [12]. Since exposed hydrophobic sites of unfolded proteins are rec ...
... and structural zinc from the enzyme leading to whole loss of functionality. Diverse forms of YADH (native, apo-I and apo-II) are differed in surface hydrophobicity; thereby, exhibiting different interactions with a chaperone molecule [12]. Since exposed hydrophobic sites of unfolded proteins are rec ...
An Application of immobilized enzymes Biosensors
... Several enzymes with differing pH and temp optima could be used together ...
... Several enzymes with differing pH and temp optima could be used together ...
Biochemistry - CPBiologyMTHS
... ENZYMES are catalysts in living things 1. protein or RNA molecule (organic contains C) 2. Speeds up chemical reactions 3. Not consumed by the reaction ...
... ENZYMES are catalysts in living things 1. protein or RNA molecule (organic contains C) 2. Speeds up chemical reactions 3. Not consumed by the reaction ...
Enzymes
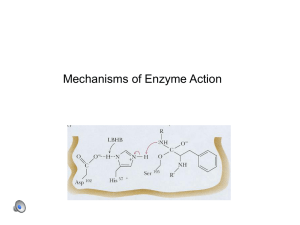
... – Irreversible inhibition: An irreversible inhibitor is a compound that inactivates an enzyme by forming covalent bonds to a particular group at the active site. Since the covalent bond is irreversible, the enzyme is rendered useless indefinitely. – Reversible inhibition: A reversible inhibitor is a ...
... – Irreversible inhibition: An irreversible inhibitor is a compound that inactivates an enzyme by forming covalent bonds to a particular group at the active site. Since the covalent bond is irreversible, the enzyme is rendered useless indefinitely. – Reversible inhibition: A reversible inhibitor is a ...
Document
... electrons through +/- charges These effects reduce G(ES*): covalent bonds, acidbase catalysis, low-barrier hydrogen bonds, and metal ion catalysis Different classes of enzymes may use different mechanisms: 1. Oxidoreductases (oxidation-reduction reactions) 2. Transferases (transfer of functional gro ...
... electrons through +/- charges These effects reduce G(ES*): covalent bonds, acidbase catalysis, low-barrier hydrogen bonds, and metal ion catalysis Different classes of enzymes may use different mechanisms: 1. Oxidoreductases (oxidation-reduction reactions) 2. Transferases (transfer of functional gro ...
Enzyme Quiz - BiologySemester57
... Complete the following table based on these results. (3) Enzyme A B C ...
... Complete the following table based on these results. (3) Enzyme A B C ...
key
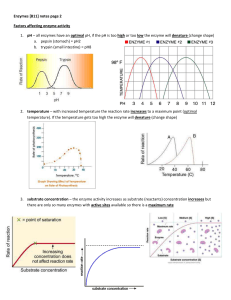
... 2. temperature – with increased temperature the reaction rate increases to a maximum point (optimal temperature). If the temperature gets too high the enzyme will denature (change shape) ...
... 2. temperature – with increased temperature the reaction rate increases to a maximum point (optimal temperature). If the temperature gets too high the enzyme will denature (change shape) ...
Reverse Multiple Sclerosis by Eating the Paleo Diet, Increasing
... phenylalanine molecule synthetically bonded with a methyl group. The methanol is what makes aspartame taste sweet. The bond holding the methyl group to phenylalanine breaks easily at temperatures higher than 85 degrees. Once separated, the methanol can travel inside any cell of the ...
... phenylalanine molecule synthetically bonded with a methyl group. The methanol is what makes aspartame taste sweet. The bond holding the methyl group to phenylalanine breaks easily at temperatures higher than 85 degrees. Once separated, the methanol can travel inside any cell of the ...
Digestive Enzymes - World of Teaching
... Maltose (a form of sugar that can be absorbed by the body ) ...
... Maltose (a form of sugar that can be absorbed by the body ) ...
Enzymes09
... biochemical reactions without altering the reaction equilibrium and the activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings. ...
... biochemical reactions without altering the reaction equilibrium and the activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings. ...
Alcohol dehydrogenase

Alcohol dehydrogenases (ADH) (EC 1.1.1.1) are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+ to NADH). In humans and many other animals, they serve to break down alcohols that otherwise are toxic, and they also participate in generation of useful aldehyde, ketone, or alcohol groups during biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+.


![Nutrition and addiction – can dietary changes assist with recovery? [3]](http://s1.studyres.com/store/data/017840792_1-ea2133c037e0fbef85c638d88f722557-300x300.png)