7.2 - Moodle
... (-) charge, and so are deflected in the opposite direction. • the gamma rays have no charge; thus, are not deflected by the field. • Be a Thinker! • Why are the beta particles deflected more than the alpha ones? ...
... (-) charge, and so are deflected in the opposite direction. • the gamma rays have no charge; thus, are not deflected by the field. • Be a Thinker! • Why are the beta particles deflected more than the alpha ones? ...
Alpha Decay
... The alpha particle is a helium nucleus (2protons, 2 neutrons) produced from the radioactive decay of heavy metals and some nuclear reaction. The high positive charge (2+) of an alpha particle causes electrical excitation and ionization of surrounding atoms. Alpha particles are the least penetr ...
... The alpha particle is a helium nucleus (2protons, 2 neutrons) produced from the radioactive decay of heavy metals and some nuclear reaction. The high positive charge (2+) of an alpha particle causes electrical excitation and ionization of surrounding atoms. Alpha particles are the least penetr ...
lecture notes - University of Chicago
... (translating to a continuous range of frequencies of electromagnetic radiation). Nuclear radiation, like radiation from individual atomic transitions, is always emitted with characteristic energies corresponding to the energy differences between nuclear energy levels. The nuclei that make up ever ...
... (translating to a continuous range of frequencies of electromagnetic radiation). Nuclear radiation, like radiation from individual atomic transitions, is always emitted with characteristic energies corresponding to the energy differences between nuclear energy levels. The nuclei that make up ever ...
Nuclear Radiation1516
... The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent of the original mass) has been converted into energy according to Einstein's equation. Fission can occur when a nucleus of a heavy atom captures a neutron, or it ...
... The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent of the original mass) has been converted into energy according to Einstein's equation. Fission can occur when a nucleus of a heavy atom captures a neutron, or it ...
Nuclear Chemistry
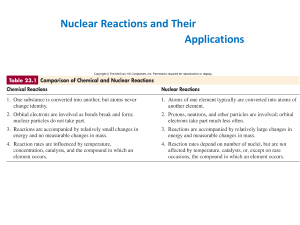
... • Protons: contribute to both the attractive force (strong force) and repelling force (charge) • Neutrons: contribute to the strong force (attractive) while having no charge. Act as the “glue” to bind the nucleus together ...
... • Protons: contribute to both the attractive force (strong force) and repelling force (charge) • Neutrons: contribute to the strong force (attractive) while having no charge. Act as the “glue” to bind the nucleus together ...
Nuclear chemistry – the study of nuclear reactions and their uses in
... Ionizing radiation is energetic enough to remove an electron from water molecule i. Harmful because most living tissue is 70% water. ii. Alpha, beta, gamma are forms of ionizing radiation. Non-ionizing radiation is generally of lower energy or slow-moving neutrons, such as radio waves. Ionizing radi ...
... Ionizing radiation is energetic enough to remove an electron from water molecule i. Harmful because most living tissue is 70% water. ii. Alpha, beta, gamma are forms of ionizing radiation. Non-ionizing radiation is generally of lower energy or slow-moving neutrons, such as radio waves. Ionizing radi ...
Chapter 25
... 1. What causes a transmutation of the nucleus to occur? 2. How are nuclear decay reaction equations balanced? 3. Do all radionuclides decay at the same rate? ...
... 1. What causes a transmutation of the nucleus to occur? 2. How are nuclear decay reaction equations balanced? 3. Do all radionuclides decay at the same rate? ...
mass numbers
... Types of Radiation (continued) positron, similar to a beta particle with a charge of 1+ and mass number of 0, and ...
... Types of Radiation (continued) positron, similar to a beta particle with a charge of 1+ and mass number of 0, and ...
File
... • Atom releases a beta particle with zero mass & negative charge • Atomic number increases (becomes new element!) ...
... • Atom releases a beta particle with zero mass & negative charge • Atomic number increases (becomes new element!) ...
Chapter 29: Nuclear Physics
... Alpha, beta, and gamma radiation penetrates to different depths in biological materials. •Alpha rays are stopped by a few cm of air or about 0.02 ...
... Alpha, beta, and gamma radiation penetrates to different depths in biological materials. •Alpha rays are stopped by a few cm of air or about 0.02 ...
Physics 102, Class 25 The Atomic Nucleus and Radioactivity
... • High-energy radiation can knock electrons off of atoms and molecules in the body. • This causes new molecules to form. Some of them are useless and some of them are harmful. • Because of the continuous background radiation that we are all exposed to all the time, life has repair mechanisms that ca ...
... • High-energy radiation can knock electrons off of atoms and molecules in the body. • This causes new molecules to form. Some of them are useless and some of them are harmful. • Because of the continuous background radiation that we are all exposed to all the time, life has repair mechanisms that ca ...
Nuclear Chemistry - Ector County ISD.
... change (since there is no change in the total number of nuclear particles), however the atomic number will increase by one (because the neutron transmutates into an additional proton). An example of this is the decay of the isotope of carbon named carbon-14 into the element nitrogen: ...
... change (since there is no change in the total number of nuclear particles), however the atomic number will increase by one (because the neutron transmutates into an additional proton). An example of this is the decay of the isotope of carbon named carbon-14 into the element nitrogen: ...
Ionizing radiation
Ionizing (or ionising in British English) radiation is radiation that carries enough energy to free electrons from atoms or molecules, thereby ionizing them. Ionizing radiation is made up of energetic subatomic particles, ions or atoms moving at relativistic speeds, and electromagnetic waves on the high-energy end of the electromagnetic spectrum.Gamma rays, X-rays, and the higher ultraviolet part of the electromagnetic spectrum are ionizing, whereas the lower ultraviolet part of the electromagnetic spectrum, visible light (including nearly all types of laser light), infrared, microwaves, and radio waves are considered non-ionizing radiation. The boundary between ionizing and non-ionizing electromagnetic radiation that occurs in the ultraviolet is not sharply defined, since different molecules and atoms ionize at different energies. Conventional definition places the boundary at a photon energy between 10 eV and 33 eV in the ultraviolet (see definition boundary section below).Typical ionizing subatomic particles from radioactivity include alpha particles, beta particles and neutrons. Almost all products of radioactive decay are ionizing because the energy of radioactive decay is typically far higher than that required to ionize. Other subatomic ionizing particles which occur naturally are muons, mesons, positrons, neutrons and other particles that constitute the secondary cosmic rays that are produced after primary cosmic rays interact with Earth's atmosphere. Cosmic rays may also produce radioisotopes on Earth (for example, carbon-14), which in turn decay and produce ionizing radiation.Cosmic rays and the decay of radioactive isotopes are the primary sources of natural ionizing radiation on Earth referred to as background radiation.In space, natural thermal radiation emissions from matter at extremely high temperatures (e.g. plasma discharge or the corona of the Sun) may be ionizing. Ionizing radiation may be produced naturally by the acceleration of charged particles by natural electromagnetic fields (e.g. lightning), although this is rare on Earth. Natural supernova explosions in space produce a great deal of ionizing radiation near the explosion, which can be seen by its effects in the glowing nebulae associated with them.Ionizing radiation can also be generated artificially using X-ray tubes, particle accelerators, and any of the various methods that produce radioisotopes artificially.Ionizing radiation is invisible and not directly detectable by human senses, so radiation detection instruments such as Geiger counters are required. However, ionizing radiation may lead to secondary emission of visible light upon interaction with matter, such as in Cherenkov radiation and radioluminescence.Ionizing radiation is applied constructively in a wide variety of fields such as medicine, research, manufacturing, construction, and many other areas, but presents a health hazard if proper measures against undesired exposure aren't followed. Exposure to ionizing radiation causes damage to living tissue, and can result in mutation, radiation sickness, cancer, and death.