Chapter 16 Notes - Mr. Julien`s Homepage
... called a mass defect. 3. Albert Einstein discovered that the defect was predictable and generated his famous equation, E = mc2, to explain the large amount of energy released from the process. 4. E is the energy released, m is the mass lost, and c is the speed of light (3 x 108 m/s). 5. The fission ...
... called a mass defect. 3. Albert Einstein discovered that the defect was predictable and generated his famous equation, E = mc2, to explain the large amount of energy released from the process. 4. E is the energy released, m is the mass lost, and c is the speed of light (3 x 108 m/s). 5. The fission ...
Physics 535 lectures notes: 1 * Sep 4th 2007
... a) Why did we observe the nuclear masses that we see? For instance, why was Helium four times more massive than hydrogen rather than twice? b) How did you bind the positively charged particles together when they should have a strong electromagnetic repulsion? c) How to explain other “radiation” emit ...
... a) Why did we observe the nuclear masses that we see? For instance, why was Helium four times more massive than hydrogen rather than twice? b) How did you bind the positively charged particles together when they should have a strong electromagnetic repulsion? c) How to explain other “radiation” emit ...
I. Ch. 21.1 Nuclear Radiation
... The process continues until unstable isotopes of one element are changed, or transformed, into stable isotopes of a different element. ...
... The process continues until unstable isotopes of one element are changed, or transformed, into stable isotopes of a different element. ...
File - Chemistry with Mr. Patmos
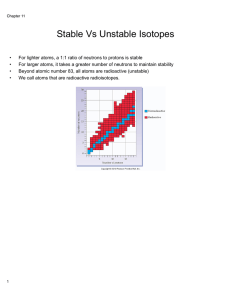
... isotopes atoms of the same element that differ in the number of neutrons. Some isotopes are stable. Some isotopes are not stable - radioactive ...
... isotopes atoms of the same element that differ in the number of neutrons. Some isotopes are stable. Some isotopes are not stable - radioactive ...
nuclear force
... • A positron is emitted from the nucleus as a proton is converted into a neutron. • The atomic number decreases by one but the mass number stays the same. ...
... • A positron is emitted from the nucleus as a proton is converted into a neutron. • The atomic number decreases by one but the mass number stays the same. ...
Nuclear - PEO Scarborough Chapter
... In beta decay, a neutron is converted into a proton and a high velocity electron is ejected to attain atomic stability. There are two types of beta decay: 1. Positive - release of positively charged particle called positron and neutrino 2. Negative - release of negatively charged particle called ele ...
... In beta decay, a neutron is converted into a proton and a high velocity electron is ejected to attain atomic stability. There are two types of beta decay: 1. Positive - release of positively charged particle called positron and neutrino 2. Negative - release of negatively charged particle called ele ...
TOPIC 5 – ATOMIC PHYSICS Radioactivity or radioactive decay:
... background radiations for the counter readings. Properties of radiations: The three types of radiations have following distinct properties. Alpha Particles (α) Nature ...
... background radiations for the counter readings. Properties of radiations: The three types of radiations have following distinct properties. Alpha Particles (α) Nature ...
Chapter 4
... Radioactivity ■ In the late 1890’s Scientists noticed some substances spontaneously emitted radiation in a process called radioactivity. This is because their nuclei is unstable ■ Rays and particles emitted are called radiation ■ Radioactive atoms undergo changes that alters their identity and allo ...
... Radioactivity ■ In the late 1890’s Scientists noticed some substances spontaneously emitted radiation in a process called radioactivity. This is because their nuclei is unstable ■ Rays and particles emitted are called radiation ■ Radioactive atoms undergo changes that alters their identity and allo ...
Chapter 19 Radioactive Material An Isotope is an element with a
... number. Produced during alpha, beta, and electron capture. Particle: 00ɣ Ex: excited nucleus ground-‐state nucleus + 00ɣ 4. Positron (+e) has the same mass of an electron, but a different charge. Wh ...
... number. Produced during alpha, beta, and electron capture. Particle: 00ɣ Ex: excited nucleus ground-‐state nucleus + 00ɣ 4. Positron (+e) has the same mass of an electron, but a different charge. Wh ...
The Atom
... When atoms emit alpha, beta or gamma radiation, it is undergoing a radioactive decay. Decay occurs due to instability within the nucleus. As the ratio of protons to neutrons becomes more skewed, the nucleus becomes more ...
... When atoms emit alpha, beta or gamma radiation, it is undergoing a radioactive decay. Decay occurs due to instability within the nucleus. As the ratio of protons to neutrons becomes more skewed, the nucleus becomes more ...
Presentation
... Colbalt 60 is commonly used as it emits very penetrating gamma radiation when their protons and neutrons change their positions in the nucleus. ...
... Colbalt 60 is commonly used as it emits very penetrating gamma radiation when their protons and neutrons change their positions in the nucleus. ...
Alpha
... have many more particles in their nucleus In some types of atom, the nucleus is unstable, and will decay into a more stable atom. This radioactive decay is completely spontaneous. It's not the same as what happens in a nuclear power station (where neutrons whizz around and hit uranium nuclei, causin ...
... have many more particles in their nucleus In some types of atom, the nucleus is unstable, and will decay into a more stable atom. This radioactive decay is completely spontaneous. It's not the same as what happens in a nuclear power station (where neutrons whizz around and hit uranium nuclei, causin ...
NUCLEAR CHEMISTRY
... One nuclear reaction is not always enough to produce a stable nuclide. A decay series is a series of radioactive nuclides produced by successive radioactive decay until a stable nuclide is reached. The heaviest nuclide of each decay series is the parent nuclide and the nuclides produced by the decay ...
... One nuclear reaction is not always enough to produce a stable nuclide. A decay series is a series of radioactive nuclides produced by successive radioactive decay until a stable nuclide is reached. The heaviest nuclide of each decay series is the parent nuclide and the nuclides produced by the decay ...
(or radioactive isotopes).
... • Gamma rays are used to kill bacteria, mould and insects in food. They are also used to kill bacteria on hospital equipment, dressings and bandages. • This is useful particularly on packaged food or on plastic items which would be damaged by heat sterilisation. • There are arguments for using cobal ...
... • Gamma rays are used to kill bacteria, mould and insects in food. They are also used to kill bacteria on hospital equipment, dressings and bandages. • This is useful particularly on packaged food or on plastic items which would be damaged by heat sterilisation. • There are arguments for using cobal ...
Content Domain III: Chemistry—Atomic Theory and
... After decaying, radioactive atoms change into other atoms. During alpha decay, the nucleus loses two protons and two neutrons. During beta decay, the nucleus gains a proton and loses a neutron. During gamma decay, the energy content of the nucleus changes but the atomic number of the element does no ...
... After decaying, radioactive atoms change into other atoms. During alpha decay, the nucleus loses two protons and two neutrons. During beta decay, the nucleus gains a proton and loses a neutron. During gamma decay, the energy content of the nucleus changes but the atomic number of the element does no ...
Ionizing radiation
Ionizing (or ionising in British English) radiation is radiation that carries enough energy to free electrons from atoms or molecules, thereby ionizing them. Ionizing radiation is made up of energetic subatomic particles, ions or atoms moving at relativistic speeds, and electromagnetic waves on the high-energy end of the electromagnetic spectrum.Gamma rays, X-rays, and the higher ultraviolet part of the electromagnetic spectrum are ionizing, whereas the lower ultraviolet part of the electromagnetic spectrum, visible light (including nearly all types of laser light), infrared, microwaves, and radio waves are considered non-ionizing radiation. The boundary between ionizing and non-ionizing electromagnetic radiation that occurs in the ultraviolet is not sharply defined, since different molecules and atoms ionize at different energies. Conventional definition places the boundary at a photon energy between 10 eV and 33 eV in the ultraviolet (see definition boundary section below).Typical ionizing subatomic particles from radioactivity include alpha particles, beta particles and neutrons. Almost all products of radioactive decay are ionizing because the energy of radioactive decay is typically far higher than that required to ionize. Other subatomic ionizing particles which occur naturally are muons, mesons, positrons, neutrons and other particles that constitute the secondary cosmic rays that are produced after primary cosmic rays interact with Earth's atmosphere. Cosmic rays may also produce radioisotopes on Earth (for example, carbon-14), which in turn decay and produce ionizing radiation.Cosmic rays and the decay of radioactive isotopes are the primary sources of natural ionizing radiation on Earth referred to as background radiation.In space, natural thermal radiation emissions from matter at extremely high temperatures (e.g. plasma discharge or the corona of the Sun) may be ionizing. Ionizing radiation may be produced naturally by the acceleration of charged particles by natural electromagnetic fields (e.g. lightning), although this is rare on Earth. Natural supernova explosions in space produce a great deal of ionizing radiation near the explosion, which can be seen by its effects in the glowing nebulae associated with them.Ionizing radiation can also be generated artificially using X-ray tubes, particle accelerators, and any of the various methods that produce radioisotopes artificially.Ionizing radiation is invisible and not directly detectable by human senses, so radiation detection instruments such as Geiger counters are required. However, ionizing radiation may lead to secondary emission of visible light upon interaction with matter, such as in Cherenkov radiation and radioluminescence.Ionizing radiation is applied constructively in a wide variety of fields such as medicine, research, manufacturing, construction, and many other areas, but presents a health hazard if proper measures against undesired exposure aren't followed. Exposure to ionizing radiation causes damage to living tissue, and can result in mutation, radiation sickness, cancer, and death.