NUCLEAR CHEMISTRY
... Most naturally occurring isotopes of elements up to atomic number 19 have stable nuclei. Elements with higher atomic number (20-83) consist of a mixture isotopes, some of which may have unstable nuclei. When the nucleus of an isotope is unstable, it is radioactive, which means that it will spontaneo ...
... Most naturally occurring isotopes of elements up to atomic number 19 have stable nuclei. Elements with higher atomic number (20-83) consist of a mixture isotopes, some of which may have unstable nuclei. When the nucleus of an isotope is unstable, it is radioactive, which means that it will spontaneo ...
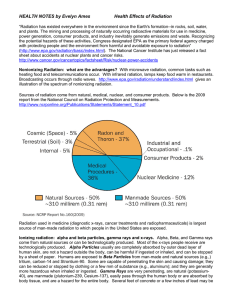
Health Effects of Radiation
... required to stop energetic gamma rays. X-Rays essentially have the same properties as Gamma rays but differ in origin; are generally lower in energy, therefore less penetrating than Gamma rays; and a few mm of lead can stop penetration of medical x-rays. How can alpha particles affect people’s heal ...
... required to stop energetic gamma rays. X-Rays essentially have the same properties as Gamma rays but differ in origin; are generally lower in energy, therefore less penetrating than Gamma rays; and a few mm of lead can stop penetration of medical x-rays. How can alpha particles affect people’s heal ...
Nuclear Chemistry
... • Example: Beta decay process is the decay of iodine- 131 into xenon131 by beta-particle emission • The mass number of the product nucleus is the same as that of the original nucleus ( they are both 131), but its atomic number has increased by 1 (54 instead of 53). This changed in atomic number, and ...
... • Example: Beta decay process is the decay of iodine- 131 into xenon131 by beta-particle emission • The mass number of the product nucleus is the same as that of the original nucleus ( they are both 131), but its atomic number has increased by 1 (54 instead of 53). This changed in atomic number, and ...
File
... • A gamma particle () or high energy photon is emitted (light) • Gamma rays have no mass, no p+ and no no so they do not alter atomic mass or atomic number. • It is PURE ENERGY which explains the large penetrating power of rays. • They are often emitted along with alpha or beta particles 230 Th ...
... • A gamma particle () or high energy photon is emitted (light) • Gamma rays have no mass, no p+ and no no so they do not alter atomic mass or atomic number. • It is PURE ENERGY which explains the large penetrating power of rays. • They are often emitted along with alpha or beta particles 230 Th ...
nuclear chemistry
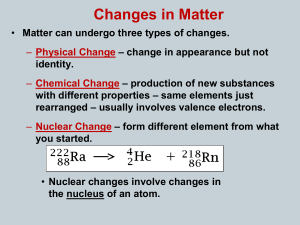
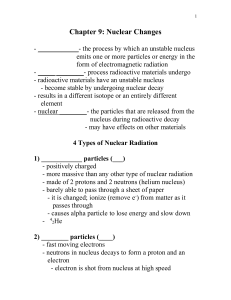
... Isotopes have the same number of protons but different numbers of neutrons NUCLEAR EQUATIONS Most nuclei are stable. Radionuclides are unstable and spontaneously emit particles and/or electromagnetic radiation U-238 is radioactive o It emits alpha particles(Helium-4 particles) When a nucleus ...
... Isotopes have the same number of protons but different numbers of neutrons NUCLEAR EQUATIONS Most nuclei are stable. Radionuclides are unstable and spontaneously emit particles and/or electromagnetic radiation U-238 is radioactive o It emits alpha particles(Helium-4 particles) When a nucleus ...
IONIZING RADIATION AND RADIONUCLIDS AS THE SOURSES …
... • Ionizing radiation is radiation with enough energy so that during an interaction with an atom, it can remove tightly bound electrons from their orbits, causing the atom to become excitated or ionized. • Ionizing radiation occurs in two forms – particles or waves. • Alpha particles is not external ...
... • Ionizing radiation is radiation with enough energy so that during an interaction with an atom, it can remove tightly bound electrons from their orbits, causing the atom to become excitated or ionized. • Ionizing radiation occurs in two forms – particles or waves. • Alpha particles is not external ...
Ionizing radiation
Ionizing (or ionising in British English) radiation is radiation that carries enough energy to free electrons from atoms or molecules, thereby ionizing them. Ionizing radiation is made up of energetic subatomic particles, ions or atoms moving at relativistic speeds, and electromagnetic waves on the high-energy end of the electromagnetic spectrum.Gamma rays, X-rays, and the higher ultraviolet part of the electromagnetic spectrum are ionizing, whereas the lower ultraviolet part of the electromagnetic spectrum, visible light (including nearly all types of laser light), infrared, microwaves, and radio waves are considered non-ionizing radiation. The boundary between ionizing and non-ionizing electromagnetic radiation that occurs in the ultraviolet is not sharply defined, since different molecules and atoms ionize at different energies. Conventional definition places the boundary at a photon energy between 10 eV and 33 eV in the ultraviolet (see definition boundary section below).Typical ionizing subatomic particles from radioactivity include alpha particles, beta particles and neutrons. Almost all products of radioactive decay are ionizing because the energy of radioactive decay is typically far higher than that required to ionize. Other subatomic ionizing particles which occur naturally are muons, mesons, positrons, neutrons and other particles that constitute the secondary cosmic rays that are produced after primary cosmic rays interact with Earth's atmosphere. Cosmic rays may also produce radioisotopes on Earth (for example, carbon-14), which in turn decay and produce ionizing radiation.Cosmic rays and the decay of radioactive isotopes are the primary sources of natural ionizing radiation on Earth referred to as background radiation.In space, natural thermal radiation emissions from matter at extremely high temperatures (e.g. plasma discharge or the corona of the Sun) may be ionizing. Ionizing radiation may be produced naturally by the acceleration of charged particles by natural electromagnetic fields (e.g. lightning), although this is rare on Earth. Natural supernova explosions in space produce a great deal of ionizing radiation near the explosion, which can be seen by its effects in the glowing nebulae associated with them.Ionizing radiation can also be generated artificially using X-ray tubes, particle accelerators, and any of the various methods that produce radioisotopes artificially.Ionizing radiation is invisible and not directly detectable by human senses, so radiation detection instruments such as Geiger counters are required. However, ionizing radiation may lead to secondary emission of visible light upon interaction with matter, such as in Cherenkov radiation and radioluminescence.Ionizing radiation is applied constructively in a wide variety of fields such as medicine, research, manufacturing, construction, and many other areas, but presents a health hazard if proper measures against undesired exposure aren't followed. Exposure to ionizing radiation causes damage to living tissue, and can result in mutation, radiation sickness, cancer, and death.