Bio 210 Cell Chemistry Lecture 8 “Glycolysis”
... In the first phase of glycolysis, 2 ATP are used to activate glucose by phosphorylation, giving fructose 1,6 bisphosphate. Fig. 2.22. This 6 C sugar is then split into two 3C molecules in preparation for the pay-off phase. In the pay-off phase the 3C sugar PGAL or phosphoglyceraldehyde is converted ...
... In the first phase of glycolysis, 2 ATP are used to activate glucose by phosphorylation, giving fructose 1,6 bisphosphate. Fig. 2.22. This 6 C sugar is then split into two 3C molecules in preparation for the pay-off phase. In the pay-off phase the 3C sugar PGAL or phosphoglyceraldehyde is converted ...
No Slide Title
... What provides the electron transport chain in cellular respiration with the energy it needs to function? ...
... What provides the electron transport chain in cellular respiration with the energy it needs to function? ...
Topic 7: METABOLISM: THERMODYNAMICS, CHEMICAL
... as well as the factors (substrate concentration, pH, temperature etc.) which impact the rate of enzyme catalyzed reactions. Energy- physico-chemical term for the capacity to do work ( work = moving a force over a distance); units are in calorie or more commonly in Joule. (note: force = mass x accele ...
... as well as the factors (substrate concentration, pH, temperature etc.) which impact the rate of enzyme catalyzed reactions. Energy- physico-chemical term for the capacity to do work ( work = moving a force over a distance); units are in calorie or more commonly in Joule. (note: force = mass x accele ...
File
... The next step in abiogenesis is the movement from monomers to polymers in order to make molecules that are capable of complex reactions or functions, like information storage for DNA, enzymatic activity for proteins, and energy storage with sugars. These polymers, along with the 4th macromolecule, l ...
... The next step in abiogenesis is the movement from monomers to polymers in order to make molecules that are capable of complex reactions or functions, like information storage for DNA, enzymatic activity for proteins, and energy storage with sugars. These polymers, along with the 4th macromolecule, l ...
i. introduction to metabolism and catabolism
... b) Heterotrophs remove these electrons and give them to more electronegative molecules, releasing the stored energy B. CATABOLISM 1. The energy released during catabolism must be stored so that it is available for anabolism (energy currency) a) High energy bonds, such as ATP b) Reducing equivalents, ...
... b) Heterotrophs remove these electrons and give them to more electronegative molecules, releasing the stored energy B. CATABOLISM 1. The energy released during catabolism must be stored so that it is available for anabolism (energy currency) a) High energy bonds, such as ATP b) Reducing equivalents, ...
Topic 7 - FSU Biology
... (4) pH- hydrogen ions may be participants (literally substrates) in enzyme catalyzed reactions; further, changes in pH may alter the ionization states of amino acid residues. Thus, it is not surprising that pH influences enzyme activity. Typically, enzymes show pH optima (fig. 6.16). (5) low molecul ...
... (4) pH- hydrogen ions may be participants (literally substrates) in enzyme catalyzed reactions; further, changes in pH may alter the ionization states of amino acid residues. Thus, it is not surprising that pH influences enzyme activity. Typically, enzymes show pH optima (fig. 6.16). (5) low molecul ...
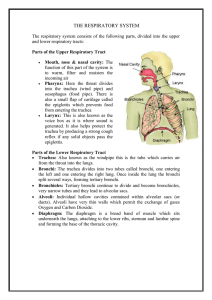
Physiology (GRPS-101) Practical notes Freshmen 2011
... function of this part of the system is to warm, filter and moisten the incoming air Pharynx: Here the throat divides into the trachea (wind pipe) and oesophagus (food pipe). There is also a small flap of cartilage called the epiglottis which prevents food from entering the trachea Larynx: This is al ...
... function of this part of the system is to warm, filter and moisten the incoming air Pharynx: Here the throat divides into the trachea (wind pipe) and oesophagus (food pipe). There is also a small flap of cartilage called the epiglottis which prevents food from entering the trachea Larynx: This is al ...
Hemoglobin as the main protein of erythrocytes. Its structure and
... their biological role. Glucose as a source of fat synthesis in liver and adipose tissue. The scheme of carbohydrate transformation into lipids. Cholesterol as a precursor for other steroids. Cholesterol synthesis: reaction sequence up to mevalonic acid formation, the idea about further stages of syn ...
... their biological role. Glucose as a source of fat synthesis in liver and adipose tissue. The scheme of carbohydrate transformation into lipids. Cholesterol as a precursor for other steroids. Cholesterol synthesis: reaction sequence up to mevalonic acid formation, the idea about further stages of syn ...
Chapter 3 Topic: Biomolecules Main concepts: •In chemistry, the
... when the helix or pleated sheet folds back on itself to form twisted or knot-like structures. This is caused by hydrogen bonding, and special bonds called disulfide bridges that form between amino acids. Quaternary structure is where multiple proteins are linked together into one large structure. He ...
... when the helix or pleated sheet folds back on itself to form twisted or knot-like structures. This is caused by hydrogen bonding, and special bonds called disulfide bridges that form between amino acids. Quaternary structure is where multiple proteins are linked together into one large structure. He ...
Macromolecules
... 1. What is the difference between a mono-, di-, and polysaccharide? 2. Provide an example of a mono-, di-, and polysaccharide. 3. What are 2 functions of carbohydrates? ...
... 1. What is the difference between a mono-, di-, and polysaccharide? 2. Provide an example of a mono-, di-, and polysaccharide. 3. What are 2 functions of carbohydrates? ...
CELL METABOLISM
... have to manufacture about 120lbs, about 50kg, of ATP per day! 5. Where does the ATP come from? What we have is a cycle, in which energy comes into the body in the form of large food molecules and is converted through many processes to ATP: - In the digestive system these large food molecules are bro ...
... have to manufacture about 120lbs, about 50kg, of ATP per day! 5. Where does the ATP come from? What we have is a cycle, in which energy comes into the body in the form of large food molecules and is converted through many processes to ATP: - In the digestive system these large food molecules are bro ...
The Central Role of Acetyl-CoA
... or a gland in one part of the body that transmit messages that affect cells in other parts of the organism. • Important hormones in human metabolism include: o Ghrelin - the hunger-stimulating hormone o Leptin - the satiety (full-feeling) hormone o Glucagon - the stored glucose releasing hormone o I ...
... or a gland in one part of the body that transmit messages that affect cells in other parts of the organism. • Important hormones in human metabolism include: o Ghrelin - the hunger-stimulating hormone o Leptin - the satiety (full-feeling) hormone o Glucagon - the stored glucose releasing hormone o I ...
Energy - Phillips Scientific Methods
... h. If no oxygen is available to the cell (anaerobic), the pyruvate will be fermented by addition of 2 H from the NADH (to alcohol + CO2 in yeast or lactic acid in muscle cells). This changes NADH back to NAD+ (oxidation) so it is available for step c above. This keeps glycolysis going! ...
... h. If no oxygen is available to the cell (anaerobic), the pyruvate will be fermented by addition of 2 H from the NADH (to alcohol + CO2 in yeast or lactic acid in muscle cells). This changes NADH back to NAD+ (oxidation) so it is available for step c above. This keeps glycolysis going! ...
Honors
... • It is readily available to form covalent bonds with other elements. • Single bonds, double bonds, triple bonds ...
... • It is readily available to form covalent bonds with other elements. • Single bonds, double bonds, triple bonds ...
Chapter 2: The Chemical Level Of Organization
... Water is the first inorganic compound listed in the table. As shown in the Functions column, water does more than just serve as a solvent for other molecules. In the form of blood, it delivers materials and heat; in the form of sweat, it cools the body via evaporation. Now let’s look at the types of ...
... Water is the first inorganic compound listed in the table. As shown in the Functions column, water does more than just serve as a solvent for other molecules. In the form of blood, it delivers materials and heat; in the form of sweat, it cools the body via evaporation. Now let’s look at the types of ...
Matrix: Citric Acid Cycle and Pyruvate Oxidation Mitochondrion A
... • Production of ATP as a result of electron transfer through carriers in the Electron Transport Chain – Electrons pass through a set of membrane-associated carriers by a series of redox reactions – Energy from electron transport powers the active transport of H+ to the intermembrane compartment of t ...
... • Production of ATP as a result of electron transfer through carriers in the Electron Transport Chain – Electrons pass through a set of membrane-associated carriers by a series of redox reactions – Energy from electron transport powers the active transport of H+ to the intermembrane compartment of t ...
SBI 4UI Test – Metabolic Processes: Cell Respiration
... Part B; Circle TRUE or FALSE on the answer sheet. F1. Chemiosmosis moves H+ into the intermembrane space of the mitochondria. F2. In the Kreb’s Cycle, malate is oxidized into fumarate. F3. Aerobic cellular respiration harvests energy from organic compounds without O2. F4. The total chemical potenti ...
... Part B; Circle TRUE or FALSE on the answer sheet. F1. Chemiosmosis moves H+ into the intermembrane space of the mitochondria. F2. In the Kreb’s Cycle, malate is oxidized into fumarate. F3. Aerobic cellular respiration harvests energy from organic compounds without O2. F4. The total chemical potenti ...
1 Respiration efficiency Respiration summary
... glucose), saving the energy required for the first glucose phosphorylation Synthesis of glycogen from glucose 6-phosphate is mediated by uridine diphosphate (a close relation of the nucleotide uracil) Costs 1 ATP per glucose 6-p to incorporate. Nonbranching residues are phosphorylised at no energeti ...
... glucose), saving the energy required for the first glucose phosphorylation Synthesis of glycogen from glucose 6-phosphate is mediated by uridine diphosphate (a close relation of the nucleotide uracil) Costs 1 ATP per glucose 6-p to incorporate. Nonbranching residues are phosphorylised at no energeti ...
Cellular Respiration Notes
... The pyruvic acid resulting from glycolysis is sent into the mitochondria for these reactions to occur. To move one molecule of pyruvic acid from the cytoplasm into a mitochondrion “costs” the cell one molecule of ATP (therefore two ATPs for a whole glucose), thus a net total of 36 ATP molecules per ...
... The pyruvic acid resulting from glycolysis is sent into the mitochondria for these reactions to occur. To move one molecule of pyruvic acid from the cytoplasm into a mitochondrion “costs” the cell one molecule of ATP (therefore two ATPs for a whole glucose), thus a net total of 36 ATP molecules per ...
Soccer Metabolic Training
... phosphocreatine (PC). With this system, as energy is released from ATP by the splitting of a phosphate group, your cells can prevent ATP depletion by reducing PC, providing more energy from ATP. This process is rapid and can be accomplished without any special structures within the cell. Although it ...
... phosphocreatine (PC). With this system, as energy is released from ATP by the splitting of a phosphate group, your cells can prevent ATP depletion by reducing PC, providing more energy from ATP. This process is rapid and can be accomplished without any special structures within the cell. Although it ...
Basal metabolic rate

Basal metabolic rate (BMR) is the minimal rate of energy expenditure per unit time by endothermic animals at rest. (McNab, B. K. 1997). On the Utility of Uniformity in the Definition of Basal Rate of Metabolism. Physiol. Zool. Vol.70; Metabolism refers to the processes that the body needs to function. Basal Metabolic Rate is the amount of energy expressed in calories that a person needs to keep the body functioning at rest. Some of those processes are breathing, blood circulation, controlling body temperature, cell growth, brain and nerve function, and contraction of muscles. Basal metabolic rate (BMR) affects the rate that a person burns calories and ultimately whether you maintain, gain, or lose weight. Your basal metabolic rate accounts for about 60 to 75% of the calories you burn every day. It is influenced by several factors.