Medical Biochemistry Review #2 By
... phosphate bonds of ATP to endergonic reactions so that they will occur spontaneously. b. The work that requires energy derived from ATP hydrolysis includes the transport of electrons down the electron transport chain. c. One half of the ATP-ADP cycle involves the generation of ATP that starts with t ...
... phosphate bonds of ATP to endergonic reactions so that they will occur spontaneously. b. The work that requires energy derived from ATP hydrolysis includes the transport of electrons down the electron transport chain. c. One half of the ATP-ADP cycle involves the generation of ATP that starts with t ...
File
... Under conditions of starvation, enzyme levels rise as proteins are degraded and amino acid carbon skeletons are used to provide energy, thus increasing the quantity of nitrogen that must be excreted. Short-term regulation of the cycle occurs principally at CPS-I, which is inactive in the absence of ...
... Under conditions of starvation, enzyme levels rise as proteins are degraded and amino acid carbon skeletons are used to provide energy, thus increasing the quantity of nitrogen that must be excreted. Short-term regulation of the cycle occurs principally at CPS-I, which is inactive in the absence of ...
Endocrinology – glucose homeostasis
... • Acts primarily on the liver, where it stimulates glycogenolysis and gluconeogenesis and thus increases hepatic glucose output. Glucagon also stimulates ketogenesis, providing an alternative fuel for those tissues that can use it and sparing glucose for those that cannot do without. • Also causes l ...
... • Acts primarily on the liver, where it stimulates glycogenolysis and gluconeogenesis and thus increases hepatic glucose output. Glucagon also stimulates ketogenesis, providing an alternative fuel for those tissues that can use it and sparing glucose for those that cannot do without. • Also causes l ...
Lecture #1 ~ Date_________
... Too much activation energy for life • Activation energy – amount of energy needed to destabilize the bonds of a molecule – moves the reaction over an “energy hill” ...
... Too much activation energy for life • Activation energy – amount of energy needed to destabilize the bonds of a molecule – moves the reaction over an “energy hill” ...
Chapter 23 - UGA Extension
... FORMULATION PROGRAM ADVANCED CAPABILITIES • RATIOS BETWEEN NUTRIENTS & RATIOS BETWEEN INGREDIENTS – HOW TO SPECIFY AMINO ACIDS AS A PERCENTAGE OF PROTEIN, THE ENERGY TO PROTEIN RATIO, OR THE CALCIUM TO PHOSPHORUS RATIO? – HOW TO SPECIFY INGREDIENT RATIOS, LIKE OYSTER SHELL TO LIMESTONE, OR CONCENTR ...
... FORMULATION PROGRAM ADVANCED CAPABILITIES • RATIOS BETWEEN NUTRIENTS & RATIOS BETWEEN INGREDIENTS – HOW TO SPECIFY AMINO ACIDS AS A PERCENTAGE OF PROTEIN, THE ENERGY TO PROTEIN RATIO, OR THE CALCIUM TO PHOSPHORUS RATIO? – HOW TO SPECIFY INGREDIENT RATIOS, LIKE OYSTER SHELL TO LIMESTONE, OR CONCENTR ...
Exam 2
... 2. ________ Which of these statements best describes kcat. A) It is equal to the maximum velocity of an enzyme catalyzed reaction. B) It is a measure of enzyme efficiency at low concentrations of substrate. C) It is a measure of the affinity of an enzyme for its substrate. D) A higher value suggests ...
... 2. ________ Which of these statements best describes kcat. A) It is equal to the maximum velocity of an enzyme catalyzed reaction. B) It is a measure of enzyme efficiency at low concentrations of substrate. C) It is a measure of the affinity of an enzyme for its substrate. D) A higher value suggests ...
Ch - Paint Valley Local Schools
... The four macromolecules are proteins, carbohydrates, lipids, and nucleic acids. 17. Which type of macromolecule do DNA and RNA belong to? They are nucleic acids. Know what each of these molecules function to do in the human body. DNA functions to provide one’s genetic code (instructions). RNA functi ...
... The four macromolecules are proteins, carbohydrates, lipids, and nucleic acids. 17. Which type of macromolecule do DNA and RNA belong to? They are nucleic acids. Know what each of these molecules function to do in the human body. DNA functions to provide one’s genetic code (instructions). RNA functi ...
The Role of Nuclear Receptor-FGF Pathways in Hormonal
... required for the generation of bile flow and excretion of lipid waste. In the gut, they facilitate absorption of dietary lipids and fat-soluble vitamins. Moreover, bile acid biosynthesis is the most significant pathway for the elimination of excess cholesterol from the body. The conversion of choles ...
... required for the generation of bile flow and excretion of lipid waste. In the gut, they facilitate absorption of dietary lipids and fat-soluble vitamins. Moreover, bile acid biosynthesis is the most significant pathway for the elimination of excess cholesterol from the body. The conversion of choles ...
Document
... •Hormones - proteins that are responsible for the regulation of many processes in organisms. Hormones are usually quite small and can be classifies as peptides (2-100 aa.) Best known protein hormones are: insulin, growth factors, etc. •Transport proteins - These proteins are transporting or store so ...
... •Hormones - proteins that are responsible for the regulation of many processes in organisms. Hormones are usually quite small and can be classifies as peptides (2-100 aa.) Best known protein hormones are: insulin, growth factors, etc. •Transport proteins - These proteins are transporting or store so ...
DNA and Translation Gene
... • Every DNA gene codes for a specific protein • Codon/anticodon match guarantees proper amino acid • Many amino acids link to make one protein ...
... • Every DNA gene codes for a specific protein • Codon/anticodon match guarantees proper amino acid • Many amino acids link to make one protein ...
Catabolism vs Anabolism
... S/(S + Km) = 20/25. So 0.8, or 4/5, or even 80% are acceptable L is half saturated when S equals the value of Km… 20 uM The ratio is 1. Since the kcats are the same, the maximal rates (for a given amount of enzyme) are the same. ...
... S/(S + Km) = 20/25. So 0.8, or 4/5, or even 80% are acceptable L is half saturated when S equals the value of Km… 20 uM The ratio is 1. Since the kcats are the same, the maximal rates (for a given amount of enzyme) are the same. ...
Question 1. Phosgene was used during the World War - IQ
... Consider half-cell A and B, draw an electrochemical cell with spontaneous reaction (write the global equation) and calculate the cell potential. Furthermore, you need to indicate: the flow of electrons, cathode and anode. (b) Metallic copper (Cu0) can be dissolved by HNO3(conc) and it is observed th ...
... Consider half-cell A and B, draw an electrochemical cell with spontaneous reaction (write the global equation) and calculate the cell potential. Furthermore, you need to indicate: the flow of electrons, cathode and anode. (b) Metallic copper (Cu0) can be dissolved by HNO3(conc) and it is observed th ...
amino acids - UniMAP Portal
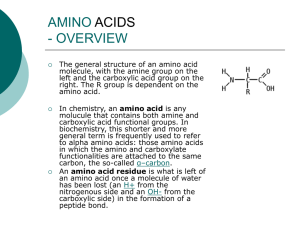
... The general structure of an amino acid molecule, with the amine group on the left and the carboxylic acid group on the right. The R group is dependent on the amino acid. In chemistry, an amino acid is any molucule that contains both amine and carboxylic acid functional groups. In biochemistry, this ...
... The general structure of an amino acid molecule, with the amine group on the left and the carboxylic acid group on the right. The R group is dependent on the amino acid. In chemistry, an amino acid is any molucule that contains both amine and carboxylic acid functional groups. In biochemistry, this ...
Chapter 9
... 4. Krebs cycle - takes the two 3 Carbon compounds from Glycolysis and extracts all Carbons and Oxygens as CO2 and Hydrogen electrons are transported by NADH/FADH2. Gain - 2 ATP + 6NADH + 2FADH2 5. Electron Transport Chain and Oxidative Phosphorylation: Move electrons through redox reactions, create ...
... 4. Krebs cycle - takes the two 3 Carbon compounds from Glycolysis and extracts all Carbons and Oxygens as CO2 and Hydrogen electrons are transported by NADH/FADH2. Gain - 2 ATP + 6NADH + 2FADH2 5. Electron Transport Chain and Oxidative Phosphorylation: Move electrons through redox reactions, create ...
09_Lecture_Presentation
... Comparing Fermentation with Anaerobic and Aerobic Respiration • All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food • In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis • The processes have different final electron acceptors: an or ...
... Comparing Fermentation with Anaerobic and Aerobic Respiration • All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food • In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis • The processes have different final electron acceptors: an or ...
Activity 6
... on this reaction. It is an inhibitor of the enzyme hexookinase which is the catalyst for the reaction. Is this effect in the same direction or opposite in direction to the effects in Question 10? ...
... on this reaction. It is an inhibitor of the enzyme hexookinase which is the catalyst for the reaction. Is this effect in the same direction or opposite in direction to the effects in Question 10? ...
Lecture 17 Expanded Genetic Code
... When you change the anticodon to CUA, the identity uniqueness is lost, and in addition to the Tyr . aaRS (archae) recognizing it, some E. coli aaRS also recognize and load the tRNA. The solution is to create a large library of tRNAs and use an in vitro selection scheme to identify an orthogonal one. ...
... When you change the anticodon to CUA, the identity uniqueness is lost, and in addition to the Tyr . aaRS (archae) recognizing it, some E. coli aaRS also recognize and load the tRNA. The solution is to create a large library of tRNAs and use an in vitro selection scheme to identify an orthogonal one. ...
16-18 Cellular respiration
... A German-British scientist, Hans Krebs, elucidated this catabolic pathway in the 1930s. The Krebs cycle, which is also known as the citric acid cycle, has eight enzyme-controlled steps that occur in the ...
... A German-British scientist, Hans Krebs, elucidated this catabolic pathway in the 1930s. The Krebs cycle, which is also known as the citric acid cycle, has eight enzyme-controlled steps that occur in the ...
ADAM
... • range in length from 11-176 amino acids • do not share significant sequence similarity with each other or with other proteins ...
... • range in length from 11-176 amino acids • do not share significant sequence similarity with each other or with other proteins ...
Test I Study Guide
... 13- Explain the role of dehydration synthesis and hydrolysis in the formation and breakdown of organic compounds 14- Compare and contrast the monomers, polymers, general structures, and biological functions for each of the four organic compounds (carbohydrates, proteins, lipids, and nucleic acids). ...
... 13- Explain the role of dehydration synthesis and hydrolysis in the formation and breakdown of organic compounds 14- Compare and contrast the monomers, polymers, general structures, and biological functions for each of the four organic compounds (carbohydrates, proteins, lipids, and nucleic acids). ...
Crossing Membranes – Passive Processes
... bilayer. E.g. O2, CO2 and steroid hormones • Other very small charged particles like water and small ions can also diffuse directly through the lipid bilayer. ...
... bilayer. E.g. O2, CO2 and steroid hormones • Other very small charged particles like water and small ions can also diffuse directly through the lipid bilayer. ...
ans - Gogarten Lab
... occupied by homologs that all function equally well, or nearly so. E. Protein space is made slightly smaller by removing all of the possibilities that cannot be synthesized or they will clog up the ribosome. ...
... occupied by homologs that all function equally well, or nearly so. E. Protein space is made slightly smaller by removing all of the possibilities that cannot be synthesized or they will clog up the ribosome. ...
09LecturePresentation
... • In the absence of O2, glycolysis couples with fermentation or anaerobic respiration to produce ATP • Anaerobic respiration uses an electron transport chain with an electron acceptor other than O2, for example sulfate • Fermentation uses phosphorylation instead of an electron transport chain to gen ...
... • In the absence of O2, glycolysis couples with fermentation or anaerobic respiration to produce ATP • Anaerobic respiration uses an electron transport chain with an electron acceptor other than O2, for example sulfate • Fermentation uses phosphorylation instead of an electron transport chain to gen ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.