Chemical Equilibrium - Shailendra Kumar Chemistry
... 2C + D was studied using an initial concentration of B which was 1.5 more that of A. But the equilibrium concentration of A and C were found to be equal. Then the Kc for the equilibrium is : (a) 4 ...
... 2C + D was studied using an initial concentration of B which was 1.5 more that of A. But the equilibrium concentration of A and C were found to be equal. Then the Kc for the equilibrium is : (a) 4 ...
Follow Along Notes - Jackson County School System
... CO2(g) + H2(g) H2O(g) + CO(g) When H2(g) is mixed with CO2(g) at 2,000 K, equilibrium is achieved according to the equation above. In one experiment, the following equilibrium concentrations were measured. [H2] = 0.20 mol/L [CO2] = 0.30 mol/L [H2O] = [CO] = 0.55 mol/L (a) What is the mole fraction ...
... CO2(g) + H2(g) H2O(g) + CO(g) When H2(g) is mixed with CO2(g) at 2,000 K, equilibrium is achieved according to the equation above. In one experiment, the following equilibrium concentrations were measured. [H2] = 0.20 mol/L [CO2] = 0.30 mol/L [H2O] = [CO] = 0.55 mol/L (a) What is the mole fraction ...
Keq = [A] [B] [C] [D]
... Only one of these values will be possible. Looking back at our data, we started with only 4.0 mol/L of H2 . If we subtract 4.7 mol/L, we will get a negative value for concentration, which is impossible. Therefore, x=4.7 is not a realistic answer, so x=1.9 mol/L. Substitute 'x =1.9 mol/L' back up int ...
... Only one of these values will be possible. Looking back at our data, we started with only 4.0 mol/L of H2 . If we subtract 4.7 mol/L, we will get a negative value for concentration, which is impossible. Therefore, x=4.7 is not a realistic answer, so x=1.9 mol/L. Substitute 'x =1.9 mol/L' back up int ...
Chapter 14: Chemical Equilibrium
... How can we determine (quantitatively) the composition of a reaction mixture when it is at a state of dynamic equilibrium? How do specific changes made to a system at equilibrium affect the equilibrium position? qualitatively understand changes ...
... How can we determine (quantitatively) the composition of a reaction mixture when it is at a state of dynamic equilibrium? How do specific changes made to a system at equilibrium affect the equilibrium position? qualitatively understand changes ...
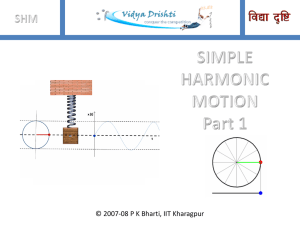
SHM Part 1 - Ask Physics
... Q. A uniform thin cylindrical disk of mass M and radius R is attached to two identical massless springs of spring constant k which are fixed to the wall as shown in the figure. The springs are attached to the axle of the disk symmetrically on either side at a distance d from its centre. The axle is ...
... Q. A uniform thin cylindrical disk of mass M and radius R is attached to two identical massless springs of spring constant k which are fixed to the wall as shown in the figure. The springs are attached to the axle of the disk symmetrically on either side at a distance d from its centre. The axle is ...
Beverley John C. Beverley IE 500/PHI 598: Ontological Engineering
... As indicated above, Thermodynamics is the study of energy, but more specifically, it is the study of energy inhering in a Thermodynamic System. Such systems will be formally defined below in the Classes section, but the intuition underlying the concept is easily grasped: they are arbitrary regions o ...
... As indicated above, Thermodynamics is the study of energy, but more specifically, it is the study of energy inhering in a Thermodynamic System. Such systems will be formally defined below in the Classes section, but the intuition underlying the concept is easily grasped: they are arbitrary regions o ...
F325 How Far How Fast test
... contains methanal, CH2O(g), and the equation for its production is shown below. O3(g) + C2H4(g) 2CH2O(g) + (a) ...
... contains methanal, CH2O(g), and the equation for its production is shown below. O3(g) + C2H4(g) 2CH2O(g) + (a) ...
Document
... – Combination of concentrations that allow Q = K – Infinite number of possible equilibrium positions • Le Châtelier’s principle – System at equilibrium (Q = K) when upset by disturbance (Q ≠ K) will shift to offset stress • System said to “shift to right” when forward reaction is dominant (Q < K) • ...
... – Combination of concentrations that allow Q = K – Infinite number of possible equilibrium positions • Le Châtelier’s principle – System at equilibrium (Q = K) when upset by disturbance (Q ≠ K) will shift to offset stress • System said to “shift to right” when forward reaction is dominant (Q < K) • ...
Thermodynamic course year 99-00
... can prevent flow of heat (adiabatic) material (a closed system) or changes in the volume or pressure. A thermodynamic state is specified by a set of thermodynamic parameters (or coordinates) necessary for the description of the system. These parameters are measurable quantities associated with the s ...
... can prevent flow of heat (adiabatic) material (a closed system) or changes in the volume or pressure. A thermodynamic state is specified by a set of thermodynamic parameters (or coordinates) necessary for the description of the system. These parameters are measurable quantities associated with the s ...
At equilibrium
... After studying this lecture you should be able to: • Understand the concepts of: the chemical equilibrium condition, dynamic equilibrium as the balance of forward and reverse reaction rates. • Know the definition of Le Chatelier’s Principle, and understand its application to the prediction of the di ...
... After studying this lecture you should be able to: • Understand the concepts of: the chemical equilibrium condition, dynamic equilibrium as the balance of forward and reverse reaction rates. • Know the definition of Le Chatelier’s Principle, and understand its application to the prediction of the di ...
Chapter 12: Chemical Equilibrium • Chemical Equilibrium
... • The equilibrium concentrations of reactants and products for a chemical reaction can be predicted using the balanced chemical equation and known equilibrium constants. – There are three basic features for the strategy used in any equilibrium calculation. • Write a balanced chemical equation for th ...
... • The equilibrium concentrations of reactants and products for a chemical reaction can be predicted using the balanced chemical equation and known equilibrium constants. – There are three basic features for the strategy used in any equilibrium calculation. • Write a balanced chemical equation for th ...
Equilibrium - chemmybear.com
... volume; more SO3) to relieve the stress. Value of Keq does not change. (b) Additional O2 disturbs the equilibrium and SO3 is formed to relieve the stress. Value of Keq does not change. (c) Increase in temperature shifts the reaction to the left to “use up” some of the added heat. Less SO3 remains. V ...
... volume; more SO3) to relieve the stress. Value of Keq does not change. (b) Additional O2 disturbs the equilibrium and SO3 is formed to relieve the stress. Value of Keq does not change. (c) Increase in temperature shifts the reaction to the left to “use up” some of the added heat. Less SO3 remains. V ...
General Concepts of Chemical Equilibrium
... between infinitesimally small amounts of products and reactants. ...
... between infinitesimally small amounts of products and reactants. ...
equilibrium
... Another example: In a closed soda bottle the dissolved CO2 gas is in equilibrium with the gas above the liquid. ...
... Another example: In a closed soda bottle the dissolved CO2 gas is in equilibrium with the gas above the liquid. ...



![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)