Lecture3_Module_19_2..
... when a stream containing variable amounts of Al3+ mixes 1:1 with a stream containing 200μM PO43- that is buffered to pH 6,5. Assume the solution is in equilibrium with AlPO4 ...
... when a stream containing variable amounts of Al3+ mixes 1:1 with a stream containing 200μM PO43- that is buffered to pH 6,5. Assume the solution is in equilibrium with AlPO4 ...
Subject:
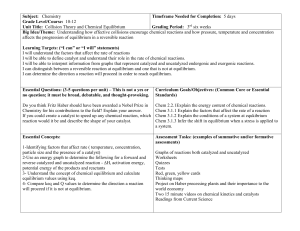
... Big Idea/Theme: Understanding how effective collisions encourage chemical reactions and how pressure, temperature and concentration affects the progression of equilibrium in a reversible reaction Learning Targets: (“I can” or “I will” statements) I will understand the factors that affect the rate of ...
... Big Idea/Theme: Understanding how effective collisions encourage chemical reactions and how pressure, temperature and concentration affects the progression of equilibrium in a reversible reaction Learning Targets: (“I can” or “I will” statements) I will understand the factors that affect the rate of ...
Physical Chemistry III
... Objective of the course: o To acquire the foundations and terminology which characterize the thermodynamic chemistry of material balances in terms of state functions. o To apply thermodynamic chemistry to the resolution of significant problems such as energy changes in chemical reactions, phase chan ...
... Objective of the course: o To acquire the foundations and terminology which characterize the thermodynamic chemistry of material balances in terms of state functions. o To apply thermodynamic chemistry to the resolution of significant problems such as energy changes in chemical reactions, phase chan ...
Big Idea 6
... • Assume reaction occurs in the forward direction. • Some strategies: – Perfect square – Quadratic equation – 5% rule (used when K is very small-compared to initial concentration) ...
... • Assume reaction occurs in the forward direction. • Some strategies: – Perfect square – Quadratic equation – 5% rule (used when K is very small-compared to initial concentration) ...
Lecture I
... When a system is at equilibrium, its state is defined entirely by the state variables, and not by the history of the system. The properties of the system can be described by an equation of state which specifies the relationship between these variables. ...
... When a system is at equilibrium, its state is defined entirely by the state variables, and not by the history of the system. The properties of the system can be described by an equation of state which specifies the relationship between these variables. ...
P2-Equilibrium Activity
... Not all chemical reactions reach completion where the limiting reactant is consumed completely. In fact, most chemical reactions that occur in living systems never reach completion. Rather, they produce some amount of product then appear to stop reacting in the forward direction, never fully consumi ...
... Not all chemical reactions reach completion where the limiting reactant is consumed completely. In fact, most chemical reactions that occur in living systems never reach completion. Rather, they produce some amount of product then appear to stop reacting in the forward direction, never fully consumi ...
Chemical Equilibrium II
... identical, thus no ____ (macroscopic) change is observed. However, individual components are actively being transformed at the microscopic level. Guldberg and Waage showed that the rate of the reaction in either direction is proportional to what they called the “active masses” of the various compone ...
... identical, thus no ____ (macroscopic) change is observed. However, individual components are actively being transformed at the microscopic level. Guldberg and Waage showed that the rate of the reaction in either direction is proportional to what they called the “active masses” of the various compone ...
Exercises Chem Eqm
... 7.1(a) K = 2.85 x 10-6; (b) ∆rGo = +240 kJ mol-1; (c) ∆rG = 0 7.4(a) Mole fractions A: 0.087, B: 0.370, C: 0.196, D: 0.348, Total: 1.001; (b) Kx – 0.33; (c) p = 0.33; (d) ∆rGo = + 2.8 x 103 J mol-1. 7.6(a) ∆rHo = +2.77 kJ mol-1, ∆rSo = -16.5 J K-1 mol-1 7.9(a) χB = 0.904, χI = 0.096 7.11(a) ∆rGo = – ...
... 7.1(a) K = 2.85 x 10-6; (b) ∆rGo = +240 kJ mol-1; (c) ∆rG = 0 7.4(a) Mole fractions A: 0.087, B: 0.370, C: 0.196, D: 0.348, Total: 1.001; (b) Kx – 0.33; (c) p = 0.33; (d) ∆rGo = + 2.8 x 103 J mol-1. 7.6(a) ∆rHo = +2.77 kJ mol-1, ∆rSo = -16.5 J K-1 mol-1 7.9(a) χB = 0.904, χI = 0.096 7.11(a) ∆rGo = – ...
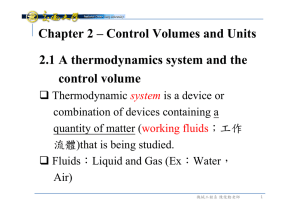
2.1 A thermodynamics system and the control volume Chapter 2
... Note:Allow heat to flow in and out. Mechanical equilibrium P will not changes with time (Variation in height is negligible) Chemical equilibrium (Chapter 16) Thermodynamic equilibrium: when a system is in equilibrium regarding all possible changes of state. 機械工程系 陳俊勳老師 ...
... Note:Allow heat to flow in and out. Mechanical equilibrium P will not changes with time (Variation in height is negligible) Chemical equilibrium (Chapter 16) Thermodynamic equilibrium: when a system is in equilibrium regarding all possible changes of state. 機械工程系 陳俊勳老師 ...
Practice with Chemical Equilibrium (Chapter 14) (Due 2/17)
... See your instructor if you have questions. Note that for these questions, the symbol "=" is used to indicate a reversible reaction. Your textbook uses a double-headed arrow. 1. Suppose that equal molar amounts of PCl3 and Cl2 are added to a 2.00 L vessel at 250 o C. The two reagents react to form PC ...
... See your instructor if you have questions. Note that for these questions, the symbol "=" is used to indicate a reversible reaction. Your textbook uses a double-headed arrow. 1. Suppose that equal molar amounts of PCl3 and Cl2 are added to a 2.00 L vessel at 250 o C. The two reagents react to form PC ...
Title - Iowa State University
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
CHM 111: General Physical Chemistry 3 Units
... empirical gas laws, Ideal Gas Equation of State, qualitative treatment of kinetic theory of gases, real gases and deviations from ideal gas laws; liquid, macroscopic properties of liquids, evaporation, vapor pressure and its variation with temperature, boiling point, heat of vaporization, Clausius-C ...
... empirical gas laws, Ideal Gas Equation of State, qualitative treatment of kinetic theory of gases, real gases and deviations from ideal gas laws; liquid, macroscopic properties of liquids, evaporation, vapor pressure and its variation with temperature, boiling point, heat of vaporization, Clausius-C ...
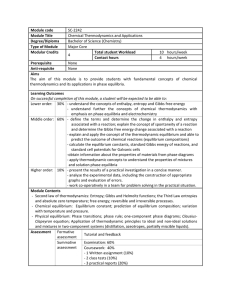
Module code SC-2242 Module Title Chemical Thermodynamics and
... -obtain information about the properties of materials from phase diagrams - apply thermodynamic concepts to understand the properties of mixtures and solution phase equilibria Higher order: 10% - present the results of a practical investigation in a concise manner. - analyse the experimental d ...
... -obtain information about the properties of materials from phase diagrams - apply thermodynamic concepts to understand the properties of mixtures and solution phase equilibria Higher order: 10% - present the results of a practical investigation in a concise manner. - analyse the experimental d ...