Chemistry 12 Worksheet 2-3 Calculations Involving the
... [A] = 0.45M, [B] = 0.63M and [C] = 0.30M. Calculate the value of the equilibrium constant for this reaction. Answer ____________________ b) At the same temperature, another equilibrium mixture is analyzed and it is found that [B] = 0.21 M and [C] = 0.70 M. From this and the information above, calcul ...
... [A] = 0.45M, [B] = 0.63M and [C] = 0.30M. Calculate the value of the equilibrium constant for this reaction. Answer ____________________ b) At the same temperature, another equilibrium mixture is analyzed and it is found that [B] = 0.21 M and [C] = 0.70 M. From this and the information above, calcul ...
AP Chem unit 13 presentation
... 5. Define the change needed to reach equilibrium, and define the equilibrium concentrations by applying the change to the initial concentrations. 6. Substitute the equilibrium concentrations into the equilibrium expression, and solve for the unknown. 7. Check your calculated equilibrium concentratio ...
... 5. Define the change needed to reach equilibrium, and define the equilibrium concentrations by applying the change to the initial concentrations. 6. Substitute the equilibrium concentrations into the equilibrium expression, and solve for the unknown. 7. Check your calculated equilibrium concentratio ...
PowerPoint Presentation - Chemical Equilibrium
... reactions considered until now have had reactants react completely to form products. These reactions “went” only in one direction. Some reactions can react in either direction. They are “reversible”. When this occurs some amount of reactant(s) will always remain in the final reaction mixture. ...
... reactions considered until now have had reactants react completely to form products. These reactions “went” only in one direction. Some reactions can react in either direction. They are “reversible”. When this occurs some amount of reactant(s) will always remain in the final reaction mixture. ...
Chapter - WTPS.org
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...
Chapter22_LEC
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...
Chemical Equilibrium - Chemistry with Mrs. Caruso Let the Bonding
... Ex. Suppose there is an equilibrium position described by the concentrations: N2+ 3H2 ⇌ 2NH3 [N2]= .399M; [H2]= 1.197M; [NH3]= .202M What will happen if 1.000 M of N2 is added to the system at constant volume? Will shift to the right. 2. Change in Pressure or Volume: Only for Gases!!!! If _____incre ...
... Ex. Suppose there is an equilibrium position described by the concentrations: N2+ 3H2 ⇌ 2NH3 [N2]= .399M; [H2]= 1.197M; [NH3]= .202M What will happen if 1.000 M of N2 is added to the system at constant volume? Will shift to the right. 2. Change in Pressure or Volume: Only for Gases!!!! If _____incre ...
Lab announcements – 2 lab quiz week before spring break
... Most chemical reactions do not go to completion. chemical equilibrium – two opposing reactions occur simultaneously at the same rate ‘equilibrium’ doesn’t necessarily mean ‘equal’ amounts of reactants and products – in fact, it usually doesn’t. Equilibrium constant – measure of this balance aA + Kc ...
... Most chemical reactions do not go to completion. chemical equilibrium – two opposing reactions occur simultaneously at the same rate ‘equilibrium’ doesn’t necessarily mean ‘equal’ amounts of reactants and products – in fact, it usually doesn’t. Equilibrium constant – measure of this balance aA + Kc ...
7.1 CHEMICAL SYSTEMS IN EQUILIBRIUM: Dynamic Equilibrium in
... For this, you need to know whether heat is given out or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is evolved):… ...
... For this, you need to know whether heat is given out or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is evolved):… ...
atomic and molecular physics using positron traps
... high annihilation rates. Many of these issues are now beginning to be addressed. The availability of efficient positron accumulators led to the development of a new method to create cold, bright low-energy positron beams [32, 33]. With energy resolution ≤ 20 meV and tunable over energies from < 100 ...
... high annihilation rates. Many of these issues are now beginning to be addressed. The availability of efficient positron accumulators led to the development of a new method to create cold, bright low-energy positron beams [32, 33]. With energy resolution ≤ 20 meV and tunable over energies from < 100 ...
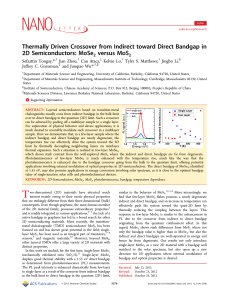
Thermally Driven Crossover from Indirect toward Direct Bandgap in
... MoSe2 and MoS2 from bulk to few-layer and to the single-layer limit. According to our DFT calculations as well as previously reported studies on MoSe2 and MoS2,21,25 these two materials possess indirect bandgap in bulk and become direct bandgap in the 2D limit. Therefore in those limits, one would e ...
... MoSe2 and MoS2 from bulk to few-layer and to the single-layer limit. According to our DFT calculations as well as previously reported studies on MoSe2 and MoS2,21,25 these two materials possess indirect bandgap in bulk and become direct bandgap in the 2D limit. Therefore in those limits, one would e ...
K eq
... No - The paper wads keep flying in both directions even after equilibrium is achieved ...
... No - The paper wads keep flying in both directions even after equilibrium is achieved ...
Minimum electrophilicity principle in Lewis acid–base complexes of
... To reinvestigate the acidity strength of some boron trihalides (BX3; X = F, Cl and Br) from theoretical point of view, two sets of Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes are formed by stronger ac ...
... To reinvestigate the acidity strength of some boron trihalides (BX3; X = F, Cl and Br) from theoretical point of view, two sets of Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes are formed by stronger ac ...
Chapter 15. Chemical Equilibrium
... However, if we start with just ammonia and no nitrogen or hydrogen, the reaction will proceed and N2 and H2 will be produced until equilibrium is achieved. No matter what the starting composition of reactants and products is, the equilibrium mixture contains the same relative concentrations of react ...
... However, if we start with just ammonia and no nitrogen or hydrogen, the reaction will proceed and N2 and H2 will be produced until equilibrium is achieved. No matter what the starting composition of reactants and products is, the equilibrium mixture contains the same relative concentrations of react ...
ch17
... If equilibrium quantities are given, we simply substitute these into the expression for Kc to calculate its value. If only some equilibrium quantities are given, we use a reaction table to calculate them and find Kc. ...
... If equilibrium quantities are given, we simply substitute these into the expression for Kc to calculate its value. If only some equilibrium quantities are given, we use a reaction table to calculate them and find Kc. ...
Mechanism of ultra low friction of multilayer graphene
... The question is then the mechanism of friction between the transfer film and the graphite surface. Does the friction occur inside the transfer film similar to the macroscopic friction of the pile of papers? If not, what is the difference in the friction mechanism between general solid materials and ...
... The question is then the mechanism of friction between the transfer film and the graphite surface. Does the friction occur inside the transfer film similar to the macroscopic friction of the pile of papers? If not, what is the difference in the friction mechanism between general solid materials and ...
Absence of photoemission from the Fermi level in potassium
... picene or coronene are doped with potassium (or sodium). It is well known from other molecular materials that particular doped phases might form while others are unstable. The most famous examples are potassium doped C60 compounds where for instance, stable K3 C60 , K4 C60 and K6 C60 phases have bee ...
... picene or coronene are doped with potassium (or sodium). It is well known from other molecular materials that particular doped phases might form while others are unstable. The most famous examples are potassium doped C60 compounds where for instance, stable K3 C60 , K4 C60 and K6 C60 phases have bee ...
Surface morphology control of immiscible polymer
... PMMA will play a dominant role in determining the initial morphology after spin-coating. Once the phase separation took place during spin coating, the PMMA-rich phase was more quickly depleted of the solvent and solidified on the hydrophilic substrate and could aggregate to form PMMArich phase layer ...
... PMMA will play a dominant role in determining the initial morphology after spin-coating. Once the phase separation took place during spin coating, the PMMA-rich phase was more quickly depleted of the solvent and solidified on the hydrophilic substrate and could aggregate to form PMMArich phase layer ...
Manipulation of powder characteristics by interactions at the solid
... attributed to increased wetability and or crystal structure defects caused by the uptake of the surfactant onto the crystal (Chiou et al., 1976). The presence of Tween 80 did not appreciably affect the flow properties (Table 3) or the dissolution rate (Fig. 5) of recrystallized SD. It can be specula ...
... attributed to increased wetability and or crystal structure defects caused by the uptake of the surfactant onto the crystal (Chiou et al., 1976). The presence of Tween 80 did not appreciably affect the flow properties (Table 3) or the dissolution rate (Fig. 5) of recrystallized SD. It can be specula ...
effect of temperature on the evolution of structure, crystallographic
... appearance of the PF practically does not change, and it looks similar to the PF after the first pass (Fig. 2b). At the same time, after the eighth pass (Fig. 2e) the orientation ( 1 2 1 1)<31 4 1>, which is close to the PF center and belongs to the pyramidal type, becomes stronger. The experimental ...
... appearance of the PF practically does not change, and it looks similar to the PF after the first pass (Fig. 2b). At the same time, after the eighth pass (Fig. 2e) the orientation ( 1 2 1 1)<31 4 1>, which is close to the PF center and belongs to the pyramidal type, becomes stronger. The experimental ...
Chemical Equilibrium
... • Equilibrium positions depend upon the initial concentrations of the reactants and products. • In an equilibrium expression, do not include pure solids (s) or liquids (l) • This is because their concentration is their density, which does not change at any given temperature. ...
... • Equilibrium positions depend upon the initial concentrations of the reactants and products. • In an equilibrium expression, do not include pure solids (s) or liquids (l) • This is because their concentration is their density, which does not change at any given temperature. ...
wiley_ch6_Chemical_Equilibrium
... T shifts reaction in direction that produces endothermic (heat absorbing) change T shifts reaction in direction that produces exothermic (heat releasing) change Changes in T change value of mass action expression at equilibrium, so K changed K depends on T T of exothermic reaction mak ...
... T shifts reaction in direction that produces endothermic (heat absorbing) change T shifts reaction in direction that produces exothermic (heat releasing) change Changes in T change value of mass action expression at equilibrium, so K changed K depends on T T of exothermic reaction mak ...
equilibrium - TeacherWeb
... EQUILIBRIUM Le Chatelier’s Principle – If a system at equilibrium is disturbed by a change in temperature, pressure or the concentration of one of the components, the system will shift its equilibrium position so as to counteract the effect of the disturbance. 3 ways that a chemical equilibrium can ...
... EQUILIBRIUM Le Chatelier’s Principle – If a system at equilibrium is disturbed by a change in temperature, pressure or the concentration of one of the components, the system will shift its equilibrium position so as to counteract the effect of the disturbance. 3 ways that a chemical equilibrium can ...
Notes - Text
... If we start with a mixture of nitrogen and hydrogen (in any proportions), the reaction will reach equilibrium with constant concentrations of nitrogen, hydrogen, and ammonia. However, if we start with just ammonia and no nitrogen or hydrogen, the reaction will proceed and N2 and H2 will be produced ...
... If we start with a mixture of nitrogen and hydrogen (in any proportions), the reaction will reach equilibrium with constant concentrations of nitrogen, hydrogen, and ammonia. However, if we start with just ammonia and no nitrogen or hydrogen, the reaction will proceed and N2 and H2 will be produced ...
20. Chemical Equilibrium
... In most of the chemical reactions we have studied so far, it appears as though all of the reactants are converted to products before a reaction stops. In truth, however, experiments show that the conversion of reactants into products is often incomplete in chemical reactions. This is the case no mat ...
... In most of the chemical reactions we have studied so far, it appears as though all of the reactants are converted to products before a reaction stops. In truth, however, experiments show that the conversion of reactants into products is often incomplete in chemical reactions. This is the case no mat ...
Topic 7.2 Equilibrium The Position of Equilibrium
... The effect of a catalyst on equilibrium Adding a catalyst speeds up a reaction by providing an alternative mechanism with a lower activation energy, thus speeding up both the forward and backward reaction rate. It shortens the time needed to attain equilibrium concentrations It has no effect ...
... The effect of a catalyst on equilibrium Adding a catalyst speeds up a reaction by providing an alternative mechanism with a lower activation energy, thus speeding up both the forward and backward reaction rate. It shortens the time needed to attain equilibrium concentrations It has no effect ...