Physical and Chemical Prop/changes
... Every substance has characteristic properties that are used to distinguish it from others. ...
... Every substance has characteristic properties that are used to distinguish it from others. ...
Irreversible Changes
... Most changes that cannot be reversed are chemical reactions where a new material is formed, and it is not possible or extremely difficult to recover the original materials. Children will experience such changes all the time in their everyday life and in the science activities they do in school, but ...
... Most changes that cannot be reversed are chemical reactions where a new material is formed, and it is not possible or extremely difficult to recover the original materials. Children will experience such changes all the time in their everyday life and in the science activities they do in school, but ...
Unit 4: Physical Properties and Changes
... Metalloid – an element that shares some properties of metals and some of non-metals Non-metal – an element that is usually a gas or brittle solid at room temperature, is not malleable or ductile, is a poor conductor of heat and electricity, and is typically not shiny Overflow can – a can used for me ...
... Metalloid – an element that shares some properties of metals and some of non-metals Non-metal – an element that is usually a gas or brittle solid at room temperature, is not malleable or ductile, is a poor conductor of heat and electricity, and is typically not shiny Overflow can – a can used for me ...
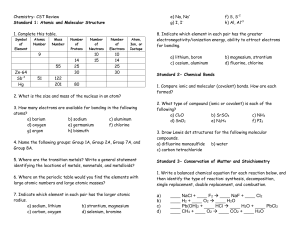
Chemistry- CST Review
... 6. How does changing the amount of gas, volume of gas, and temperature affect the gas pressure? For Q’s #9-14, name the gas law and show all your work. 7. The pressure on 2.00 L of anesthetic gas changes from 100 kPa to 40 kPa. What will be the new volume if the temperature remains constant? 8. If a ...
... 6. How does changing the amount of gas, volume of gas, and temperature affect the gas pressure? For Q’s #9-14, name the gas law and show all your work. 7. The pressure on 2.00 L of anesthetic gas changes from 100 kPa to 40 kPa. What will be the new volume if the temperature remains constant? 8. If a ...
LABORATORY FACILITIES IN THE
... various materials. The TMA system provides measurements of penetration, expansion, contraction, and extension of materials as a function of temperature from -170 to 325°C, while the TGA system measures weight changes as a function of time or temperature from ambient to l000°C. In the DTA and DSC sys ...
... various materials. The TMA system provides measurements of penetration, expansion, contraction, and extension of materials as a function of temperature from -170 to 325°C, while the TGA system measures weight changes as a function of time or temperature from ambient to l000°C. In the DTA and DSC sys ...
7A SCIENCE FINAL REVIEW - MERRICK 7th SCIENCE REVIEW
... ___ Define matter. ___ Describe the difference between atoms and molecules. ___ Define elements, compounds, and mixtures. ___ Recognize elements from compounds if given the chemical symbol or a model. ___ Describe the difference between a chemical and physical property of matter, give examples of ea ...
... ___ Define matter. ___ Describe the difference between atoms and molecules. ___ Define elements, compounds, and mixtures. ___ Recognize elements from compounds if given the chemical symbol or a model. ___ Describe the difference between a chemical and physical property of matter, give examples of ea ...
Unit 13 Worksheet Answers
... Name_______________________________________period_______unit 13 worksheet kinetics and equilibrium 1) What is meant by the term "rate of reaction"? How fast a reaction occurs 2) It is found that a 10oC increase in temperature roughly doubles the rate of many chemical reactions. If a reaction takes 2 ...
... Name_______________________________________period_______unit 13 worksheet kinetics and equilibrium 1) What is meant by the term "rate of reaction"? How fast a reaction occurs 2) It is found that a 10oC increase in temperature roughly doubles the rate of many chemical reactions. If a reaction takes 2 ...
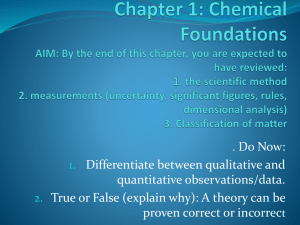
Chapter 1: Chemical Foundations
... Making observations Qualitative Observations - observations made by using your senses (does not involve a number) Quantitative Observations - observations made by measuring (involves a number and a unit) Formulating hypotheses hypothesis - a possible explanation for an observation Perfor ...
... Making observations Qualitative Observations - observations made by using your senses (does not involve a number) Quantitative Observations - observations made by measuring (involves a number and a unit) Formulating hypotheses hypothesis - a possible explanation for an observation Perfor ...
Diagnosis Test: EDEXCEL ADDITIONAL SCIENCE Biology
... QWC Suggested marking guidance (Total 6 marks) Marks awarded for this answer will be determined by the Quality of Written Communication (QWC) as well as the standard of the scientific response. Teachers should apply a ‘best-fit’ approach to the marking. ...
... QWC Suggested marking guidance (Total 6 marks) Marks awarded for this answer will be determined by the Quality of Written Communication (QWC) as well as the standard of the scientific response. Teachers should apply a ‘best-fit’ approach to the marking. ...
Le Chatelier`s Principle Quiz Answer Key
... 9. What factors alter the equilibrium position in chemical reactions? 10. Describe Le Chatelier's principle. 11. If more reactant is added to an equilibrium system, what happens to Keq (assume the temperature is not changed)? 12. How is changing the concentration of a reactant in a reaction related ...
... 9. What factors alter the equilibrium position in chemical reactions? 10. Describe Le Chatelier's principle. 11. If more reactant is added to an equilibrium system, what happens to Keq (assume the temperature is not changed)? 12. How is changing the concentration of a reactant in a reaction related ...
constant power parallel heating cable
... CONSTANT POWER PARALLEL HEATING CABLE - SPCx S The structural technology of this heating wire allows to solve installation problems in a practical and safe way and at a reduced price. It is the ideal solution in situations requiring flexibility of use, an easy assembly and a quick performance. Thank ...
... CONSTANT POWER PARALLEL HEATING CABLE - SPCx S The structural technology of this heating wire allows to solve installation problems in a practical and safe way and at a reduced price. It is the ideal solution in situations requiring flexibility of use, an easy assembly and a quick performance. Thank ...
Many thermal and chemical reactions occur during the roasting
... Sucrose is the principle sugar in coffee. The melting point of pure crystalline sucrose is in the 320-392 degrees F with 370 degrees F most commonly accepted. Degradation of dry sucrose can occur as low as 194 degrees F. and begins with the cleavage of the glycosidic bond followed by condensation an ...
... Sucrose is the principle sugar in coffee. The melting point of pure crystalline sucrose is in the 320-392 degrees F with 370 degrees F most commonly accepted. Degradation of dry sucrose can occur as low as 194 degrees F. and begins with the cleavage of the glycosidic bond followed by condensation an ...
CC-80 art 6
... Thermogravimetry (TG) studies the change (gain or loss) of a sample mass as a function of temperature and/or time. The measurements of these changes are made using a thermobalance in which the tests are accomplished according to a programed heating rate in a suitable enclosed system with a controlle ...
... Thermogravimetry (TG) studies the change (gain or loss) of a sample mass as a function of temperature and/or time. The measurements of these changes are made using a thermobalance in which the tests are accomplished according to a programed heating rate in a suitable enclosed system with a controlle ...
بسم الله الرحمن الرحيم
... 1 – A mixture of a gas in a gas can only form a solution 2 – Enthalpy is the heat of reaction at constant pressure 3 – The solute particles can be seperated from colloides by filtration 4 – Potential energy equals m . g . h 5 – Molarity is an intensive property. 6 – There are 3 significant figures i ...
... 1 – A mixture of a gas in a gas can only form a solution 2 – Enthalpy is the heat of reaction at constant pressure 3 – The solute particles can be seperated from colloides by filtration 4 – Potential energy equals m . g . h 5 – Molarity is an intensive property. 6 – There are 3 significant figures i ...
Chapter 1_chemh
... of matter present. (melting point, boiling point, etc) ●Physical Property: characteristic that can be observed or measured without changing the identity of the substance. (melting, boiling, etc) oPhysical Change: change that does not involve a change in the identity of the substance. (cutting, melti ...
... of matter present. (melting point, boiling point, etc) ●Physical Property: characteristic that can be observed or measured without changing the identity of the substance. (melting, boiling, etc) oPhysical Change: change that does not involve a change in the identity of the substance. (cutting, melti ...
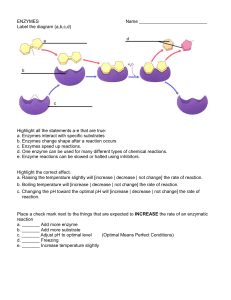
ENZYMES
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
Name__________________________________ Block______
... 1. Air is a(n) (element, compound, mixture). 2. Salt water is a(n) (mixture, compound, element). 3. (Breaking, Melting, Burning) is an example of a chemical change. 4. (Lemonade, Iced tea, Water) is not a mixture. 5. (Density, Burning, Color) is not a physical property. 6. Iron exists as a (solid, l ...
... 1. Air is a(n) (element, compound, mixture). 2. Salt water is a(n) (mixture, compound, element). 3. (Breaking, Melting, Burning) is an example of a chemical change. 4. (Lemonade, Iced tea, Water) is not a mixture. 5. (Density, Burning, Color) is not a physical property. 6. Iron exists as a (solid, l ...
On a Universal Tendency in Nature to the Dissipation
... consequences which follow from Carnot's proposition, that there is an absolute waste of mechanical energy available to man when heat is allowed to pass from one body to another at a lower temperature, by any means not fulfilling his criterion of a “perfect thermo-dynamic engine,” established, on a n ...
... consequences which follow from Carnot's proposition, that there is an absolute waste of mechanical energy available to man when heat is allowed to pass from one body to another at a lower temperature, by any means not fulfilling his criterion of a “perfect thermo-dynamic engine,” established, on a n ...
6d reversible and irreversible changes
... where temperature is produced. This decreased. We use a is an thermometer to irreversible measure temperature. change. ...
... where temperature is produced. This decreased. We use a is an thermometer to irreversible measure temperature. change. ...
Theoretical Calculation of Enthalpy of reactions involved in PZ
... Heat of absorption is a very important thermodynamic parameter in CO2 removal using amine solvent solutions in post combustion temperature swing processes. The temperature dependency of the heat of absorption for such processes can be calculated from the theoretical equilibrium constants [1] for eac ...
... Heat of absorption is a very important thermodynamic parameter in CO2 removal using amine solvent solutions in post combustion temperature swing processes. The temperature dependency of the heat of absorption for such processes can be calculated from the theoretical equilibrium constants [1] for eac ...
THERMODYNAMICS III
... monoclinic sulphur, and vapour, are in equilibrium is at 5x10-7 bar and 368.4 K. At this point the densities of rhombic and monoclinic sulphur are 2070 kg m-3 and 1960 kg m-3 respectively, and the enthalpy change in the transition is 819 J mol-1. Estimate the temperature of the triple point between ...
... monoclinic sulphur, and vapour, are in equilibrium is at 5x10-7 bar and 368.4 K. At this point the densities of rhombic and monoclinic sulphur are 2070 kg m-3 and 1960 kg m-3 respectively, and the enthalpy change in the transition is 819 J mol-1. Estimate the temperature of the triple point between ...
CH225h - Oregon State chemistry
... The rms speeds are inversely proportional to the square root of mass, so the ratio of Ar speed / He speed is ≈ sqrt (4/40) ≈ 0.3 (b) If Ar, He, and Xe are mixed with equal partial pressures in a gas sample, which will effuse most rapidly through a small hole in the container ? Explain briefly. He. E ...
... The rms speeds are inversely proportional to the square root of mass, so the ratio of Ar speed / He speed is ≈ sqrt (4/40) ≈ 0.3 (b) If Ar, He, and Xe are mixed with equal partial pressures in a gas sample, which will effuse most rapidly through a small hole in the container ? Explain briefly. He. E ...
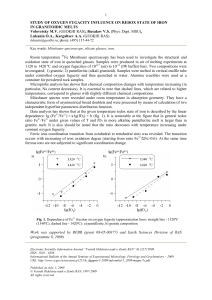
Study of oxygen fugacity influence on redox state of iron in
... under controlled oxygen fugacity and then quenched in water. Alumina crucibles were used as a container for powdered rock samples. Microprobe analysis has shown that chemical composition changes with temperature increasing (in particular, Na content decreases). It is essential to note that dashed li ...
... under controlled oxygen fugacity and then quenched in water. Alumina crucibles were used as a container for powdered rock samples. Microprobe analysis has shown that chemical composition changes with temperature increasing (in particular, Na content decreases). It is essential to note that dashed li ...
AP Chemistry Review Packet 1 CO2(g) + H2(g) « H2O(g) + CO(g
... An experiment is to be performed to determine the standard molar enthalpy of neutralization of a strong acid by a strong base. Standard school laboratory equipment and a supply of standardized 1.00-molar HCl and standardized 1.00-molar NaOH are available. (a) What equipment would be needed? (b) Wha ...
... An experiment is to be performed to determine the standard molar enthalpy of neutralization of a strong acid by a strong base. Standard school laboratory equipment and a supply of standardized 1.00-molar HCl and standardized 1.00-molar NaOH are available. (a) What equipment would be needed? (b) Wha ...
Glossary
... where temperature is produced. This decreased. We use a is an thermometer to irreversible measure temperature. change. ...
... where temperature is produced. This decreased. We use a is an thermometer to irreversible measure temperature. change. ...