Name__________________________________ Block______
... 9. Mixtures can only be separated by chemical means. 10. Chemical changes produce new substances with new chemical properties. 11. A substance in the solid phase can be changed into the liquid phase. 12. Elements are composed of a single type of atom. 13. Solutions, elements and compounds are all un ...
... 9. Mixtures can only be separated by chemical means. 10. Chemical changes produce new substances with new chemical properties. 11. A substance in the solid phase can be changed into the liquid phase. 12. Elements are composed of a single type of atom. 13. Solutions, elements and compounds are all un ...
Ch. 1-- Matter and Change
... They all take on the same qualities and for our purposes become one ...
... They all take on the same qualities and for our purposes become one ...
Cosmetology Learning Module 12
... Is a change in the chemical and physical properties of a substance by a chemical reaction that creates a new substance or substances The result of a chemical reaction that creates new chemicals that have new chemical and ...
... Is a change in the chemical and physical properties of a substance by a chemical reaction that creates a new substance or substances The result of a chemical reaction that creates new chemicals that have new chemical and ...
The retrospect of the science and the thermodynamics
... Calculating the work requirement and heat transfer to a series of chemical engineering process, a plants. Prediction or estimation the composition in each phase at equilibria states. ...
... Calculating the work requirement and heat transfer to a series of chemical engineering process, a plants. Prediction or estimation the composition in each phase at equilibria states. ...
classification of chemical reactions
... # of reactants = # of products # of atoms on the right = # of atoms on the left Mg + O2 MgO balanced or unbalanced? ...
... # of reactants = # of products # of atoms on the right = # of atoms on the left Mg + O2 MgO balanced or unbalanced? ...
Discover Chemical Changes - gk-12
... with before and after chemical changes so that students can make the chemical changes happen themselves or at least make observations of chemical changes that have happened at each station. I have listed above 9 possible chemical changes that can be used. Students should move from station to station ...
... with before and after chemical changes so that students can make the chemical changes happen themselves or at least make observations of chemical changes that have happened at each station. I have listed above 9 possible chemical changes that can be used. Students should move from station to station ...
SCIENCE 9
... has its own distinct properties and cannot be broken down into simpler substances by means of a chemical change. COMPOUNDS- are pure substances that are made up of two or more elements chemically combined together. Compounds can be broken down into elements again by chemical means DALTON’S ATOMIC TH ...
... has its own distinct properties and cannot be broken down into simpler substances by means of a chemical change. COMPOUNDS- are pure substances that are made up of two or more elements chemically combined together. Compounds can be broken down into elements again by chemical means DALTON’S ATOMIC TH ...
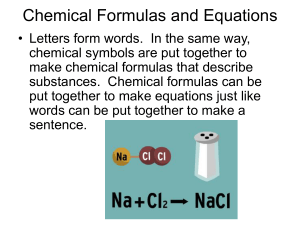
Chemical Formulas and Equations
... Chemical Formulas and Equations • Letters form words. In the same way, chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a ...
... Chemical Formulas and Equations • Letters form words. In the same way, chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a ...
53 word equations
... the '+' sign means 'and' the '' sign means 'changed into' the reactants come in front of the arrow the products come after the arrow ...
... the '+' sign means 'and' the '' sign means 'changed into' the reactants come in front of the arrow the products come after the arrow ...
Reactions Unit Plan
... B. Apply the Law of Conservation of Mass to writing and balancing chemical equations. (MOCLE 1.1.I.a, b) 1. Represent a chemical equation in words and formulas. 2. Balance a chemical equation using the Law of Conservation of ...
... B. Apply the Law of Conservation of Mass to writing and balancing chemical equations. (MOCLE 1.1.I.a, b) 1. Represent a chemical equation in words and formulas. 2. Balance a chemical equation using the Law of Conservation of ...
Physical and Chemical Changes
... In A Chemical Change.... • A substance’s chemical formula is changed and the substance is changed into something completely new. • It no longer has the physical or chemical properties it had before. ...
... In A Chemical Change.... • A substance’s chemical formula is changed and the substance is changed into something completely new. • It no longer has the physical or chemical properties it had before. ...
Chapter 5 – Chemical Reactions
... Particle size – the smaller the particles the faster the reaction (example – dust explosion) Higher temperature – the higher the temperature the faster the reaction Increase concentration of solution (a more concentrated acid will react faster than a dilute acid) Add a catalyst – a catalyst is a che ...
... Particle size – the smaller the particles the faster the reaction (example – dust explosion) Higher temperature – the higher the temperature the faster the reaction Increase concentration of solution (a more concentrated acid will react faster than a dilute acid) Add a catalyst – a catalyst is a che ...
SNC2D – Science 10 Tuesday April 26th, 2010 Mr. Sourlis and Mr
... 3. When a chemical reaction takes place, the total mass of the products is always a. Greater than the total mass of the reactants b. Less than the total mass of the reactants c. Equal to the total mass of the reactants d. Dependent on the type of reaction e. Impossible to determine 4. What is anothe ...
... 3. When a chemical reaction takes place, the total mass of the products is always a. Greater than the total mass of the reactants b. Less than the total mass of the reactants c. Equal to the total mass of the reactants d. Dependent on the type of reaction e. Impossible to determine 4. What is anothe ...
Introduction to Chemical Reactions
... Chemical Equations are balanced to show the same number of atoms of each element on each side. The Law of Conservation of Mass says that atoms won’t be created or destroyed in a chemical reaction. That is why you have to balance chemical equations! ...
... Chemical Equations are balanced to show the same number of atoms of each element on each side. The Law of Conservation of Mass says that atoms won’t be created or destroyed in a chemical reaction. That is why you have to balance chemical equations! ...
Matter Change
... Occurrence can be indicated by changes in temperature, color, odor, & physical state Also known as a chemical change Chemical properties can only be observed when a substance undergoes a chemical change ...
... Occurrence can be indicated by changes in temperature, color, odor, & physical state Also known as a chemical change Chemical properties can only be observed when a substance undergoes a chemical change ...
Unit 1 - Measurement Atomic Theory
... (i) Boiling Point, Melting Point, Malleability, Ductility, Specific Gravity, luster, vapor pressure, etc. ...
... (i) Boiling Point, Melting Point, Malleability, Ductility, Specific Gravity, luster, vapor pressure, etc. ...
Matter in Chemistry
... heat to boil an egg, it causes a chemical reaction between the yolk and the white that leaves a green film around the yolk. That film is iron sulfide, caused by iron in the yolk reacting with hydrogen sulfide in the white (it won't hurt you to eat it, and the egg will taste the same). ...
... heat to boil an egg, it causes a chemical reaction between the yolk and the white that leaves a green film around the yolk. That film is iron sulfide, caused by iron in the yolk reacting with hydrogen sulfide in the white (it won't hurt you to eat it, and the egg will taste the same). ...
Balancing Equations
... • Chemical Reaction: One or more reactants change into one or more products • Reactant: A substance present at the start of a reaction • Product: A substance produced in a chemical reaction • Chemical Equation: An expression representing a chemical reaction; the formulas of the reactants (on the lef ...
... • Chemical Reaction: One or more reactants change into one or more products • Reactant: A substance present at the start of a reaction • Product: A substance produced in a chemical reaction • Chemical Equation: An expression representing a chemical reaction; the formulas of the reactants (on the lef ...
Chemistry 2011-2012
... SC1 Students will analyze the nature of matter and its classifications. SC1a. Relate the role of nuclear fusion in producing essentially all elements heavier than helium. SC1b. Identify substances based on chemical and physical properties. SC2 Students will relate how the Law of Conservation of Matt ...
... SC1 Students will analyze the nature of matter and its classifications. SC1a. Relate the role of nuclear fusion in producing essentially all elements heavier than helium. SC1b. Identify substances based on chemical and physical properties. SC2 Students will relate how the Law of Conservation of Matt ...
Ch. 3 - Chemical Reactions
... Evolution of heat and light Formation of a gas Formation of a precipitate Color change ...
... Evolution of heat and light Formation of a gas Formation of a precipitate Color change ...
Science Notes on Physical and Chemical Properties
... The appearance may change, but you still have the same substance as before – can be reversed and no energy is produced Example – Tear a piece of paper into 10-15 pieces. The shape and size have changed, but its still paper Example – Change of state = physical change…add energy to ice and you get a l ...
... The appearance may change, but you still have the same substance as before – can be reversed and no energy is produced Example – Tear a piece of paper into 10-15 pieces. The shape and size have changed, but its still paper Example – Change of state = physical change…add energy to ice and you get a l ...
Al-Shifa pharmaceutical factory

The Al-Shifa (الشفاء, Arabic for ""healing"") pharmaceutical factory in Khartoum North, Sudan, was constructed between 1992 and 1996 with components imported from the United States, Sweden, Italy, Switzerland, Germany, India, and Thailand. It was officially opened on July 12, 1997.The industrial complex was composed of around four buildings. It was the largest pharmaceutical factory in Khartoum and employed over 300 workers, producing medicine both for human and veterinary use.The factory was destroyed in 1998 by a missile attack launched by the United States government, killing one employee and wounding eleven. Critics of the attack have estimated that up to tens of thousands of Sudanese civilians died throughout Sudan as the supply of necessary drugs was cut off. The U.S. government stated several reasons for its attack: The alleged use of the factory for the processing of VX nerve agent. For alleged ties between the owners of the plant and al-Qaeda.These justifications for the bombing were disputed by the owners of the plant, the Sudanese government, and other governments.