
Chemical Properties - Michigan State University
... what is occurring in the lab. How has the sugar changed? (asked after the physical AND chemical change) Is the sugar still present? How are the physical and chemical changes different? How would you classify a physical change? What about a chemical change? I want to discuss and ask a question also a ...
... what is occurring in the lab. How has the sugar changed? (asked after the physical AND chemical change) Is the sugar still present? How are the physical and chemical changes different? How would you classify a physical change? What about a chemical change? I want to discuss and ask a question also a ...
Lecture Notes
... The standard unit of measurement for heat is the calorie (cal). The calorie is defined as the quantity of heat necessary to raise the temperature on one gram of water 1o centigrade at 15o centigrade. In metabolic processes the calorie is often too small a unit, the kilocalorie(Kcal) is used. The kil ...
... The standard unit of measurement for heat is the calorie (cal). The calorie is defined as the quantity of heat necessary to raise the temperature on one gram of water 1o centigrade at 15o centigrade. In metabolic processes the calorie is often too small a unit, the kilocalorie(Kcal) is used. The kil ...
Skill Sheet 19-B Chemical Formulas
... number for bromine (Br) is 1-. In order for the oxidation numbers of this compound add up to zero, one atom of aluminum must combine with three atoms of bromine: ...
... number for bromine (Br) is 1-. In order for the oxidation numbers of this compound add up to zero, one atom of aluminum must combine with three atoms of bromine: ...
Chapter 2 Matter
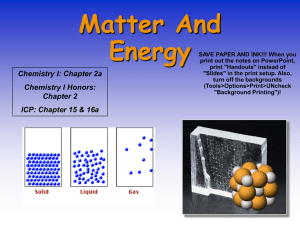
... Liquids have no shape, but they do have a volume. Solids are rigid and have a definite shape and volume. ...
... Liquids have no shape, but they do have a volume. Solids are rigid and have a definite shape and volume. ...
Unit 1 Cycle 2: Interactions and Energy
... identity), they only change in their motion and spatial configuration, this is a physical change. When antacid and vinegar are mixed, they undergo a reaction. The atoms of the calcium carbonate and acetic acid particles rearrange into different spatial configurations, forming calcium acetate, carbon ...
... identity), they only change in their motion and spatial configuration, this is a physical change. When antacid and vinegar are mixed, they undergo a reaction. The atoms of the calcium carbonate and acetic acid particles rearrange into different spatial configurations, forming calcium acetate, carbon ...
File - Kentucky Recreation and Park Society
... fertilizers, pesticides, turf growth regulators, bio-stimulants and wetting agents. Responsible to maintain, repair, and calibrate all chemical application equipment; maintain and keep the chemical inventory current including the Safety Data Sheets (SDS) library and all chemical application records ...
... fertilizers, pesticides, turf growth regulators, bio-stimulants and wetting agents. Responsible to maintain, repair, and calibrate all chemical application equipment; maintain and keep the chemical inventory current including the Safety Data Sheets (SDS) library and all chemical application records ...
Chapter One Powerpoint - Geneva Area City Schools
... • solid state, matter has definite volume and definite shape. • liquid state, matter has a definite volume but an indefinite shape. • gas state, matter has neither definite volume nor definite shape. • Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, ...
... • solid state, matter has definite volume and definite shape. • liquid state, matter has a definite volume but an indefinite shape. • gas state, matter has neither definite volume nor definite shape. • Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, ...
Student Activity PDF - TI Education
... 3. For each word equation given on page 2.10, use the Chemical Balance tool on page 2.11 to balance the equation and record it in the table. First, write the balanced equation using the element symbols. Record the number of atoms of each element in the reactant (left side) and the products (right si ...
... 3. For each word equation given on page 2.10, use the Chemical Balance tool on page 2.11 to balance the equation and record it in the table. First, write the balanced equation using the element symbols. Record the number of atoms of each element in the reactant (left side) and the products (right si ...
Laboratory Chemicals.. - Oklahoma State University
... health and safety concerns associated with these chemicals, specific chemical management protocols should be implemented and followed. For the laboratory setting, these protocols should be outlined in the chemical hygiene plan. Identification of these chemicals and their hazards is the first step in ...
... health and safety concerns associated with these chemicals, specific chemical management protocols should be implemented and followed. For the laboratory setting, these protocols should be outlined in the chemical hygiene plan. Identification of these chemicals and their hazards is the first step in ...
Chapter 1 - Manual Science Chemistry/Physics
... List examples of the branches of chemistry Compare and contrast basic research applied research, and technological development. Main Ideas: Chemistry is the study of matter and its processes. o A chemical is any substance that has a definite composition. o What questions does chemistry seek ...
... List examples of the branches of chemistry Compare and contrast basic research applied research, and technological development. Main Ideas: Chemistry is the study of matter and its processes. o A chemical is any substance that has a definite composition. o What questions does chemistry seek ...
UNIT 7 Lecture Notes
... number, you can get yourself out of that situation by doubling ALL the coefficients in the equation. (if you end up with .25 you can get yourself out of that situation by quadrupling all coefficients) 3) Take your time. Balancing is a skill that takes practice but with enough patience an answer will ...
... number, you can get yourself out of that situation by doubling ALL the coefficients in the equation. (if you end up with .25 you can get yourself out of that situation by quadrupling all coefficients) 3) Take your time. Balancing is a skill that takes practice but with enough patience an answer will ...
Matter and Energy
... Chemistry & Matter • We can explore the MACROSCOPIC world — what we can see — • to understand the PARTICULATE worlds we cannot see. • We write SYMBOLS to describe these worlds. ...
... Chemistry & Matter • We can explore the MACROSCOPIC world — what we can see — • to understand the PARTICULATE worlds we cannot see. • We write SYMBOLS to describe these worlds. ...
COUNTING ATOMS
... CHEMICAL EQUATIONS • Some equations have a coefficient. • Coefficients are numbers that appear before elements in a chemical equation that change the number of reactants or products. • Example: • H2 + O2 H20 • The correct way to write this equation is: • 2H2 + O2 2H2O • The coefficients change ...
... CHEMICAL EQUATIONS • Some equations have a coefficient. • Coefficients are numbers that appear before elements in a chemical equation that change the number of reactants or products. • Example: • H2 + O2 H20 • The correct way to write this equation is: • 2H2 + O2 2H2O • The coefficients change ...
I. States of Matter
... Dissolve as much of the mixture as possible Add water and stir Filter the mixture so that the soluble salt will be obtained in the filtrate and the insoluble chalk powder will be the residue on the filter paper Place the dry mixture into a beaker Dry out the filter paper to keep the dry chalk powder ...
... Dissolve as much of the mixture as possible Add water and stir Filter the mixture so that the soluble salt will be obtained in the filtrate and the insoluble chalk powder will be the residue on the filter paper Place the dry mixture into a beaker Dry out the filter paper to keep the dry chalk powder ...
Describing Matter Chapter 2:2 Physical and Chemical Properties
... easy to undo • Chemical Properties~ a property of matter that describes a substance based on its ability to change into a new substance with different properties • Chemical Change~ a change that occurs when one or more substances are changed into entirely new substances with different properties; ca ...
... easy to undo • Chemical Properties~ a property of matter that describes a substance based on its ability to change into a new substance with different properties • Chemical Change~ a change that occurs when one or more substances are changed into entirely new substances with different properties; ca ...
KUT 101/2 – General Chemistry Practical I
... • Differentiate the primary and secondary standards. • Differentiate the equivalence and end-points. • Choose suitable indicators for acid-base titration. • Determine the amount of acid in an unknown. • Know a quantitative technique of volumetric analysis. • Understand the definition of BOD (Biochem ...
... • Differentiate the primary and secondary standards. • Differentiate the equivalence and end-points. • Choose suitable indicators for acid-base titration. • Determine the amount of acid in an unknown. • Know a quantitative technique of volumetric analysis. • Understand the definition of BOD (Biochem ...
PowerPoint - Balancing Equations
... 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. (reduced) ...
... 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. (reduced) ...
Chemical Reactions
... 1. Determine the correct formulas for all 4. Balance the elements one at a time by the reactants and products. using coefficients. When no coefficient is written, it is assumed to be 1. Begin by 2. Write the skeleton equation by placing the formulas for the reactants on the left balancing elements t ...
... 1. Determine the correct formulas for all 4. Balance the elements one at a time by the reactants and products. using coefficients. When no coefficient is written, it is assumed to be 1. Begin by 2. Write the skeleton equation by placing the formulas for the reactants on the left balancing elements t ...
Chemistry
... 12. Understand an MSDS for any compound. 13. Follow all safety rules, actions, protocols with respect to lab behavior, chemical handling and response in an emergency. 14. Use UV, IR, NMR and mass spectrometry data to deduce the structure of an organic compound. 15. Use the internet to retrieve infor ...
... 12. Understand an MSDS for any compound. 13. Follow all safety rules, actions, protocols with respect to lab behavior, chemical handling and response in an emergency. 14. Use UV, IR, NMR and mass spectrometry data to deduce the structure of an organic compound. 15. Use the internet to retrieve infor ...
[Mg] +2[ S ]-2
... 11. Adding ice cubes to hot chocolate so it cools down faster not a chemical reaction 12. The smell that is given off from a stink bomb chemical reaction Using the 5 indicators of chemical reactions explain how you can determine whether a chemical reaction has taken place or not in the scenario belo ...
... 11. Adding ice cubes to hot chocolate so it cools down faster not a chemical reaction 12. The smell that is given off from a stink bomb chemical reaction Using the 5 indicators of chemical reactions explain how you can determine whether a chemical reaction has taken place or not in the scenario belo ...
Properties and Changes Reading Assignment Name: Chemistry 2
... into sheets without breaking. But gold is more malleable than copper. Hardness, color, conductivity, and malleability are examples of physical properties. A physical property is a quality or condition of a substance that can be observed or measured without changing the substance’s composition. What ...
... into sheets without breaking. But gold is more malleable than copper. Hardness, color, conductivity, and malleability are examples of physical properties. A physical property is a quality or condition of a substance that can be observed or measured without changing the substance’s composition. What ...
Example - cloudfront.net
... a) Balance elements that appear in more than one compound __________ (NH4)2CO3 NH3 + CO2 + H2O b) Balance __________________ as though they are one item as long as the ion stays together as a group on each side of the arrow. Al + CuSO4 Al2(SO4)3 + Cu c) If you can’t seem to get it balanced, ____ ...
... a) Balance elements that appear in more than one compound __________ (NH4)2CO3 NH3 + CO2 + H2O b) Balance __________________ as though they are one item as long as the ion stays together as a group on each side of the arrow. Al + CuSO4 Al2(SO4)3 + Cu c) If you can’t seem to get it balanced, ____ ...
chemical reaction
... taken place requires evidence that one or more substances have undergone a change in identity. Absolute proof of such a change can be provided only by chemical analysis of the products. ...
... taken place requires evidence that one or more substances have undergone a change in identity. Absolute proof of such a change can be provided only by chemical analysis of the products. ...
Al-Shifa pharmaceutical factory

The Al-Shifa (الشفاء, Arabic for ""healing"") pharmaceutical factory in Khartoum North, Sudan, was constructed between 1992 and 1996 with components imported from the United States, Sweden, Italy, Switzerland, Germany, India, and Thailand. It was officially opened on July 12, 1997.The industrial complex was composed of around four buildings. It was the largest pharmaceutical factory in Khartoum and employed over 300 workers, producing medicine both for human and veterinary use.The factory was destroyed in 1998 by a missile attack launched by the United States government, killing one employee and wounding eleven. Critics of the attack have estimated that up to tens of thousands of Sudanese civilians died throughout Sudan as the supply of necessary drugs was cut off. The U.S. government stated several reasons for its attack: The alleged use of the factory for the processing of VX nerve agent. For alleged ties between the owners of the plant and al-Qaeda.These justifications for the bombing were disputed by the owners of the plant, the Sudanese government, and other governments.

















![[Mg] +2[ S ]-2](http://s1.studyres.com/store/data/014450548_1-468f3af464a09baae245d79fadf97d41-300x300.png)


