HYDROGEN PEROXIDE 20%-50%
... Handling: Operate in a well-ventilated area. Keep away fro heat sources. Keep away from incompatible products. Prevent all contact with organics. Use equipment and containers which are compatible with the substance. Before all operations, passivate the piping, circuits and vessels. Never return unus ...
... Handling: Operate in a well-ventilated area. Keep away fro heat sources. Keep away from incompatible products. Prevent all contact with organics. Use equipment and containers which are compatible with the substance. Before all operations, passivate the piping, circuits and vessels. Never return unus ...
7.5.9 Compare physical properties of matter to the chemical property
... Density is a property that describes the relationship between the mass of a material and its volume Substances that are denser contain MORE matter in a given volume ...
... Density is a property that describes the relationship between the mass of a material and its volume Substances that are denser contain MORE matter in a given volume ...
Unit 1. Materials: Formulating Matter A. How do chemists describe
... 31. You melted and burned paraffin wax in the Lab Investigating Matter. Write the chemical formula of paraffin wax given its model below. (Note: The carbon and hydrogen atoms are smaller than in the key so that this molecule can fit on the page.) ...
... 31. You melted and burned paraffin wax in the Lab Investigating Matter. Write the chemical formula of paraffin wax given its model below. (Note: The carbon and hydrogen atoms are smaller than in the key so that this molecule can fit on the page.) ...
Rinse 30001
... The product may be harmful if it is inhaled or swallowed. POTENTIAL HEALTH EFFECTS: CODE OF HAZARD STATEMENTS: Physical hazards None. Health Hazards H303-may be harmful if swallowed. H312Harmful in contact with skin. H319-Causes serious eye irritation. H333-May be harmful if inhaled. Environmental h ...
... The product may be harmful if it is inhaled or swallowed. POTENTIAL HEALTH EFFECTS: CODE OF HAZARD STATEMENTS: Physical hazards None. Health Hazards H303-may be harmful if swallowed. H312Harmful in contact with skin. H319-Causes serious eye irritation. H333-May be harmful if inhaled. Environmental h ...
Answer key
... a. Salt is mixed with water c. A paper boat is made by paper cutting b. Wax is melted d. A wax candle is burnt 17. Which of the following is not a chemical change? a. Burning of log of wood c. Curdling of milk b. Chopping of trees d. Heating of milk 18. On addition of baking soda to vinegar, we come ...
... a. Salt is mixed with water c. A paper boat is made by paper cutting b. Wax is melted d. A wax candle is burnt 17. Which of the following is not a chemical change? a. Burning of log of wood c. Curdling of milk b. Chopping of trees d. Heating of milk 18. On addition of baking soda to vinegar, we come ...
8.5DF: Chemical Formulas and Equations
... Cooking with Chemical Formulas and Equations To help students learn more about chemical formulas and equations, work with your child to explain how equations are similar to a recipe that might be used while cooking. Interestingly, there are many different ways that chemical reactions and chemical eq ...
... Cooking with Chemical Formulas and Equations To help students learn more about chemical formulas and equations, work with your child to explain how equations are similar to a recipe that might be used while cooking. Interestingly, there are many different ways that chemical reactions and chemical eq ...
I Examen I Trim Science
... 2- Describe the two types of research: basic and applied and examples of them. Research: Basic: help us with the understanding of how natural world operates. Ex. Rain, plants growing, volcanoes. Applied: focuses on developing these applications. Ex. Shampoo, Tosty food, Panadol. ...
... 2- Describe the two types of research: basic and applied and examples of them. Research: Basic: help us with the understanding of how natural world operates. Ex. Rain, plants growing, volcanoes. Applied: focuses on developing these applications. Ex. Shampoo, Tosty food, Panadol. ...
3 CO 2(g)
... Properties of original substance disappear as new substances with different properties are formed Change in chemical composition Cannot return to original form Can be detected through – energy changes (temperature), change in color, emission of gas, solid formed ...
... Properties of original substance disappear as new substances with different properties are formed Change in chemical composition Cannot return to original form Can be detected through – energy changes (temperature), change in color, emission of gas, solid formed ...
balancing chemical equations worksheet
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
Section 2 Types of Chemical Reactions Chapter 8
... • Balance the different types of atoms one at a time. • First balance the atoms of elements that are combined and that appear only once on each side of the equation. • Balance polyatomic ions that appear on both sides of the equation as single units. • Balance H atoms and O atoms after atoms of all ...
... • Balance the different types of atoms one at a time. • First balance the atoms of elements that are combined and that appear only once on each side of the equation. • Balance polyatomic ions that appear on both sides of the equation as single units. • Balance H atoms and O atoms after atoms of all ...
chemical reaction - MRS. STOTTS CHEMISTRY
... in the form of light and heat. example: combustion of hydrogen ...
... in the form of light and heat. example: combustion of hydrogen ...
Chemical Equation
... Polyatomic Anions • Polyatomic anions have more than one atom. • A nonmetal plus oxygen or oxygen and hydrogen. – Sometimes called an “oxyanion.” ...
... Polyatomic Anions • Polyatomic anions have more than one atom. • A nonmetal plus oxygen or oxygen and hydrogen. – Sometimes called an “oxyanion.” ...
Honors Chapter 2
... Matter can be a gas, a liquid, or a solid. Gases have no fixed shape or volume. Gases can be compressed to form liquids. Liquids have no shape, but they do have a volume. Solids are rigid and have a definite shape and volume. ...
... Matter can be a gas, a liquid, or a solid. Gases have no fixed shape or volume. Gases can be compressed to form liquids. Liquids have no shape, but they do have a volume. Solids are rigid and have a definite shape and volume. ...
Describing Chemical Reactions
... Chemical equations use chemical formulas and other symbols instead of words to summarize a reaction. All chemical equations have a common structure. A chemical equation tells you the substances you start with in a reaction and the substances you get at the end. The substances you have at the beginni ...
... Chemical equations use chemical formulas and other symbols instead of words to summarize a reaction. All chemical equations have a common structure. A chemical equation tells you the substances you start with in a reaction and the substances you get at the end. The substances you have at the beginni ...
Chemistry Note PowerPoint
... • The space is huge compared to the amount of space taken by the nucleus. • It symbolizes where electrons are LIKELY to be. • An electrons movement is related to its energy level, or the specific amount of energy that it has. ...
... • The space is huge compared to the amount of space taken by the nucleus. • It symbolizes where electrons are LIKELY to be. • An electrons movement is related to its energy level, or the specific amount of energy that it has. ...
Types of Chemical Reactions Name_________________________
... An Introduction to Types of Chemical Reactions The purpose of this Internet assignment is to provide you with an independent learning opportunity to learn about the different types of chemical reactions. The website address for this assignment is www.ric.edu/ptiskus/reactions. On the website you wil ...
... An Introduction to Types of Chemical Reactions The purpose of this Internet assignment is to provide you with an independent learning opportunity to learn about the different types of chemical reactions. The website address for this assignment is www.ric.edu/ptiskus/reactions. On the website you wil ...
21:3 Classifying Chemical Reactions
... Yeast is a microscopic, one-celled organism belonging to the group of organisms called fungi. There are many kinds of yeasts, some of them of great importance to humans. Yeast is necessary to make leavened bread, beer, and cheese. It is rich in B vitamins; a form of yeast called brewer's yeast is us ...
... Yeast is a microscopic, one-celled organism belonging to the group of organisms called fungi. There are many kinds of yeasts, some of them of great importance to humans. Yeast is necessary to make leavened bread, beer, and cheese. It is rich in B vitamins; a form of yeast called brewer's yeast is us ...
File
... other substances. The substances that go into a chemical reaction are the reactants. The substances produced by a chemical reaction are the products. The properties of the products are different from those of the reactants. Changes in properties that might occur in a chemical reaction are shown in F ...
... other substances. The substances that go into a chemical reaction are the reactants. The substances produced by a chemical reaction are the products. The properties of the products are different from those of the reactants. Changes in properties that might occur in a chemical reaction are shown in F ...
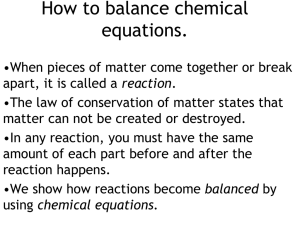
How to balance chemical equations.
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
Matter - Clayton State University
... - A combination of two or more pure substances Examples grains of rice and wheat cereal and sugar salt and sand - Components of a mixture can be separated by physical means (filtration, distillation, the use of magnet for metals) ...
... - A combination of two or more pure substances Examples grains of rice and wheat cereal and sugar salt and sand - Components of a mixture can be separated by physical means (filtration, distillation, the use of magnet for metals) ...
Unit 2 matter - Kowenscience.com
... enough to be seen and handled – physical properties can be observed by the human senses (unaided) ...
... enough to be seen and handled – physical properties can be observed by the human senses (unaided) ...
Student Expectation
... Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are called “valence electrons” and h ...
... Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are called “valence electrons” and h ...
Al-Shifa pharmaceutical factory

The Al-Shifa (الشفاء, Arabic for ""healing"") pharmaceutical factory in Khartoum North, Sudan, was constructed between 1992 and 1996 with components imported from the United States, Sweden, Italy, Switzerland, Germany, India, and Thailand. It was officially opened on July 12, 1997.The industrial complex was composed of around four buildings. It was the largest pharmaceutical factory in Khartoum and employed over 300 workers, producing medicine both for human and veterinary use.The factory was destroyed in 1998 by a missile attack launched by the United States government, killing one employee and wounding eleven. Critics of the attack have estimated that up to tens of thousands of Sudanese civilians died throughout Sudan as the supply of necessary drugs was cut off. The U.S. government stated several reasons for its attack: The alleged use of the factory for the processing of VX nerve agent. For alleged ties between the owners of the plant and al-Qaeda.These justifications for the bombing were disputed by the owners of the plant, the Sudanese government, and other governments.