jyvaskla2 - School of Chemistry
... The topological atom The partitioning in real space of molecules into atoms by using these interatomic surfaces is a fundamental, quantum mechanically rigorous, method. It allows properties to be calculated for proper open systems, where exchange, e.g. with charge may occur between atoms. The proper ...
... The topological atom The partitioning in real space of molecules into atoms by using these interatomic surfaces is a fundamental, quantum mechanically rigorous, method. It allows properties to be calculated for proper open systems, where exchange, e.g. with charge may occur between atoms. The proper ...
Balance this equation:
... The diagram shows iron oxide, Fe2O3, and carbon monoxide, CO reacting to form iron and carbon dioxide. Which of the following is the correct full balanced chemical equation for the reaction depicted? ...
... The diagram shows iron oxide, Fe2O3, and carbon monoxide, CO reacting to form iron and carbon dioxide. Which of the following is the correct full balanced chemical equation for the reaction depicted? ...
jyvaskla2 - School of Chemistry
... The topological atom The partitioning in real space of molecules into atoms by using these interatomic surfaces is a fundamental, quantum mechanically rigorous, method. It allows properties to be calculated for proper open systems, where exchange, e.g. with charge may occur between atoms. The proper ...
... The topological atom The partitioning in real space of molecules into atoms by using these interatomic surfaces is a fundamental, quantum mechanically rigorous, method. It allows properties to be calculated for proper open systems, where exchange, e.g. with charge may occur between atoms. The proper ...
Dr. Atiya Abbasi Lecture 04_ IEC_ 16 Jan.ppt
... Thus if a protein has no net charge at a certain pH (also known as isoelectric point pI) it will not interact with the charged medium. However, at a pH above its isoelectric point, a protein will bind to a positively charged medium or anion exchanger and, at a pH below its pI, the protein will behin ...
... Thus if a protein has no net charge at a certain pH (also known as isoelectric point pI) it will not interact with the charged medium. However, at a pH above its isoelectric point, a protein will bind to a positively charged medium or anion exchanger and, at a pH below its pI, the protein will behin ...
Slide 1
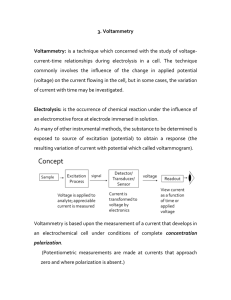
... and go into solution as magnesium ions. The electrons will be left behind on the magnesium In a very short time, there will be a build-up of electrons on the magnesium, and it will be surrounded in the solution by a layer of positive ions (Helmholtz double layer). This produces a potential defferenc ...
... and go into solution as magnesium ions. The electrons will be left behind on the magnesium In a very short time, there will be a build-up of electrons on the magnesium, and it will be surrounded in the solution by a layer of positive ions (Helmholtz double layer). This produces a potential defferenc ...
Chemistry Unit 5 Test Review The Mole and Balancing Equations
... 2. Which quantity is equivalent to 39 grams of LiF? 3. What is the mass in grams of 1.00 mole of O 2 gas? 4. What is the total number of moles contained in 115 grams of C 2 H5 OH? 5. What is the total mass in grams of 0.75 mole of SO 2 ? 6. Two alcohols that are used in our everyday lives are rubbin ...
... 2. Which quantity is equivalent to 39 grams of LiF? 3. What is the mass in grams of 1.00 mole of O 2 gas? 4. What is the total number of moles contained in 115 grams of C 2 H5 OH? 5. What is the total mass in grams of 0.75 mole of SO 2 ? 6. Two alcohols that are used in our everyday lives are rubbin ...
Fall.2008.Week9.Lesson.2 - reich
... • Combustion means burning and fire. What two things does fire require? O2 and something to burn. We normally burn hydrocarbons (Hydro=H, Carbon = C therefore stuff made up of H and C). • The products are always CO2 and H2O. • Methane and Oxygen burn write the equation. • ___CH4+ ___O2 ___ CO2 + _ ...
... • Combustion means burning and fire. What two things does fire require? O2 and something to burn. We normally burn hydrocarbons (Hydro=H, Carbon = C therefore stuff made up of H and C). • The products are always CO2 and H2O. • Methane and Oxygen burn write the equation. • ___CH4+ ___O2 ___ CO2 + _ ...
g moles molarity
... In a STRONG acid the anion can not provide enough electrostatic attraction to hold onto the H+ (proton) In a WEAK acid the anion is very charge dense and holds onto the H+ ...
... In a STRONG acid the anion can not provide enough electrostatic attraction to hold onto the H+ (proton) In a WEAK acid the anion is very charge dense and holds onto the H+ ...
hc1(8)notes
... continued • The next step in writing a correct chemical equation is to replace the names of the reactants and products with appropriate symbols and formulas. • A formula equation represents the reactants and products of a chemical reaction by their symbols or formulas. • example: The formula equatio ...
... continued • The next step in writing a correct chemical equation is to replace the names of the reactants and products with appropriate symbols and formulas. • A formula equation represents the reactants and products of a chemical reaction by their symbols or formulas. • example: The formula equatio ...
Reaction types and Stoichiometry
... g of sulfuric acid to produce potassium sulfate and water? A B C D ...
... g of sulfuric acid to produce potassium sulfate and water? A B C D ...
Describing Chemical Reactions
... place requires evidence that one or more substances have undergone a change in identity ...
... place requires evidence that one or more substances have undergone a change in identity ...
Chapter 4: Properties of Gases
... C6H12O6(s) in a bomb calorimeter (constant volume) is 47.0 kJ. Then for one mole of glucose, the quantity of heat evolved (in kJ) is (according to the equation): C6H12O6 (s) + 6O2 (g) 6CO2 (g) + 6H2O (ℓ); H = ? (a) 2.82x103 ...
... C6H12O6(s) in a bomb calorimeter (constant volume) is 47.0 kJ. Then for one mole of glucose, the quantity of heat evolved (in kJ) is (according to the equation): C6H12O6 (s) + 6O2 (g) 6CO2 (g) + 6H2O (ℓ); H = ? (a) 2.82x103 ...
How to Balance Chemical Equations
... inventory on that side of the chemical equation. Repeat the process until total number of atoms for each element perfectly matches on both sides of the chemical equation. ...
... inventory on that side of the chemical equation. Repeat the process until total number of atoms for each element perfectly matches on both sides of the chemical equation. ...
Unit 2.7: Periodic Table Group1 Group2 Li Be Na Mg K Ca Rb Sr Cs
... Group 2 metals have higher melting temperature than group1 metals in the same period. This is because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements i ...
... Group 2 metals have higher melting temperature than group1 metals in the same period. This is because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements i ...
Diffusion current - Prof Dr Hisham E Abdellatef
... b- Internal standards or Pilot ion method: This method is based on the fact that the relative diffusion current constants I are independent of the particular capillary used, provided the nature and concentration Of supporting electrolyte and the temperature are kept constant. Hence , upon determinin ...
... b- Internal standards or Pilot ion method: This method is based on the fact that the relative diffusion current constants I are independent of the particular capillary used, provided the nature and concentration Of supporting electrolyte and the temperature are kept constant. Hence , upon determinin ...
Chapter 10
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
Chapter 10
... Balanced Chemical Equations Skeleton equations do not reflect the fact that matter is conserved during a reaction. An equation must reflect that the same number of each kind of atom on both sides of the arrow. This is called a balanced chemical equation. To balance a chemical equation, use coef ...
... Balanced Chemical Equations Skeleton equations do not reflect the fact that matter is conserved during a reaction. An equation must reflect that the same number of each kind of atom on both sides of the arrow. This is called a balanced chemical equation. To balance a chemical equation, use coef ...
Building the sense of math in physics activities
... prey detect each other suddenly at the same location. The prey would have to accelerate faster than the predator in order to escape. The predator would accelerate more slowly than the prey (it's larger) but attain a higher velocity, as in the graph shown. If the prey has ventured 4 seconds away from ...
... prey detect each other suddenly at the same location. The prey would have to accelerate faster than the predator in order to escape. The predator would accelerate more slowly than the prey (it's larger) but attain a higher velocity, as in the graph shown. If the prey has ventured 4 seconds away from ...
half-reactions - Clayton State University
... Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq) Zn is oxidized (oxidation number changes from 0 to +2) Cu is reduced (oxidation number changes from +2 to 0) The oxidation half-reaction is: Zn(s) → Zn2+(aq) + 2eThe reduction half-reaction is: Cu2+(aq) + 2e- → Cu(s) ...
... Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq) Zn is oxidized (oxidation number changes from 0 to +2) Cu is reduced (oxidation number changes from +2 to 0) The oxidation half-reaction is: Zn(s) → Zn2+(aq) + 2eThe reduction half-reaction is: Cu2+(aq) + 2e- → Cu(s) ...
I CAN write Chemical formulas
... 1. Write the oxidation number above each element. 2. Cross the oxidation numbers and write the oxidation number (without plus or minus) of one element as the subscript of the other element. 3. Reduce the subscripts (number of atoms) to their simplest form, if needed. WHAT IS THE CHEMICAL FORMULA FO ...
... 1. Write the oxidation number above each element. 2. Cross the oxidation numbers and write the oxidation number (without plus or minus) of one element as the subscript of the other element. 3. Reduce the subscripts (number of atoms) to their simplest form, if needed. WHAT IS THE CHEMICAL FORMULA FO ...
Inorganic Chemistry Basics
... Chelation refers to coordination of two or more donor atoms from a single ligand to a central metal ion The resulting complex is characterized by an unusual thermodynamic stability The gain in stability upon chelation is usually ascribed to a significant gain in entropy (however: this is not always ...
... Chelation refers to coordination of two or more donor atoms from a single ligand to a central metal ion The resulting complex is characterized by an unusual thermodynamic stability The gain in stability upon chelation is usually ascribed to a significant gain in entropy (however: this is not always ...
Ch 11 Chemical Reactions
... We can’t remember them all, but most fall into one of several categories. We will learn the 5 major types, and given the reactants, predict products for a given type For some, we will be able to: c) predict whether or not they will happen at all. How? Recognize them by their reactants ...
... We can’t remember them all, but most fall into one of several categories. We will learn the 5 major types, and given the reactants, predict products for a given type For some, we will be able to: c) predict whether or not they will happen at all. How? Recognize them by their reactants ...
Chapter 7
... • Increasing the temperature of a substance causes its particles to move faster, on average. • Particles that move faster are both more likely to collide and more likely to react. • If the number of collisions that produce reactions increases, then the reaction rate increases ...
... • Increasing the temperature of a substance causes its particles to move faster, on average. • Particles that move faster are both more likely to collide and more likely to react. • If the number of collisions that produce reactions increases, then the reaction rate increases ...
Balancing reaction equations, oxidation state, and reduction
... Determining Oxidation Number of Elements & Molecules 1. In uncombined or free elements (not ionized), each atom has an oxidation number of 0. E.g., all of the atoms in these molecules: H2, Na, S8, O2, P4. 2. In simple ions (i.e., charged species which contain only one atom), the oxidation number is ...
... Determining Oxidation Number of Elements & Molecules 1. In uncombined or free elements (not ionized), each atom has an oxidation number of 0. E.g., all of the atoms in these molecules: H2, Na, S8, O2, P4. 2. In simple ions (i.e., charged species which contain only one atom), the oxidation number is ...
Double layer forces

Double layer forces occur between charged objects across liquids, typically water. This force acts over distances that are comparable to the Debye length, which is on the order of one to a few tenths of nanometers. The strength of these forces increases with the magnitude of the surface charge density (or the electrical surface potential). For two similarly charged objects, this force is repulsive and decays exponentially at larger distances, see figure. For unequally charged objects and eventually at shorted distances, these forces may also be attractive. The theory due to Derjaguin, Landau, Verwey, and Overbeek (DLVO) combines such double layer forces together with Van der Waals forces in order to estimate the actual interaction potential between colloidal particles.An electrical double layer develops near charged surfaces (or another charged objects) in aqueous solutions. Within this double layer, the first layer corresponds to the charged surface. These charges may originate from tightly adsorbed ions, dissociated surface groups, or substituted ions within the crystal lattice. The second layer corresponds to the diffuse layer, which contains the neutralizing charge consisting of accumulated counterions and depleted coions. The resulting potential profile between these two objects leads to differences in the ionic concentrations within the gap between these objects with respect to the bulk solution. These differences generate an osmotic pressure, which generates a force between these objects.These forces are easily experienced when hands are washed with soap. Adsorbing soap molecules make the skin negatively charged, and the slippery feeling is caused by the strongly repulsive double layer forces. These forces are further relevant in many colloidal or biological systems, and may be responsible for their stability, formation of colloidal crystals, or their rheological properties.