PowerPoint for Cornell Notes
... In the Redox Reaction diagram, sodium (Na) is being oxidized and chlorine (Cl) is being reduced. The term "oxidation", with its obvious root from the word "oxygen", assumes that oxygen has an oxidation number of -2. WHY -2?? BECAUSE Oxygen must gain 2 electrons in order to fill its shell ...
... In the Redox Reaction diagram, sodium (Na) is being oxidized and chlorine (Cl) is being reduced. The term "oxidation", with its obvious root from the word "oxygen", assumes that oxygen has an oxidation number of -2. WHY -2?? BECAUSE Oxygen must gain 2 electrons in order to fill its shell ...
Chapter 2 Chemical Reactions
... 4) If you have polyatomic ions that show up on both sides of the equations, replace them with a variable to make it easier. – Let X rep SO4 5) When dealing with O2 sometimes you have an odd number on one side. Put a ½ in front of the O2. Then balance and then multiply both sides by 2 6) Double-Check ...
... 4) If you have polyatomic ions that show up on both sides of the equations, replace them with a variable to make it easier. – Let X rep SO4 5) When dealing with O2 sometimes you have an odd number on one side. Put a ½ in front of the O2. Then balance and then multiply both sides by 2 6) Double-Check ...
How to Balance Chemical Equations
... to be written for all compounds/elements. Make an atom inventory on each side (reactant or product) for all elements involved in the chemical reaction. Select the element that has different number of atoms from one side to another. Find the least common factors for the two numbers. ...
... to be written for all compounds/elements. Make an atom inventory on each side (reactant or product) for all elements involved in the chemical reaction. Select the element that has different number of atoms from one side to another. Find the least common factors for the two numbers. ...
Unit 3C Standards for Quiz
... calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structure 1. The Periodic Table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates ...
... calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structure 1. The Periodic Table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates ...
lecture 13
... BALANCING EQUATIONS: The same number of each type of element must occur on the left (BEFORE the reaction) and on the right (AFTER the reaction) ...
... BALANCING EQUATIONS: The same number of each type of element must occur on the left (BEFORE the reaction) and on the right (AFTER the reaction) ...
Chemical Equations & Reactions
... An equation must be balanced. It must have the same number of atoms of the same kind on both sides. ...
... An equation must be balanced. It must have the same number of atoms of the same kind on both sides. ...
What is Matter PowerPoint
... or to change into one or more new substances. • The inability of a substance to change is also a chemical ...
... or to change into one or more new substances. • The inability of a substance to change is also a chemical ...
CHEMISTry is life - World of Teaching
... • Mol Day- Last Thursday, October 23, Beginning at 6:02a.m. and ending at 6:02p.m. Get it? Avogadro's number is 6.02 x 1023 • Chemistry Jokes There are hundreds on the internet- Here is one link: http://library.thinkquest.org/10429/gather/jokes.htm Q. What is the dieter's element? A. Nobelium • Poem ...
... • Mol Day- Last Thursday, October 23, Beginning at 6:02a.m. and ending at 6:02p.m. Get it? Avogadro's number is 6.02 x 1023 • Chemistry Jokes There are hundreds on the internet- Here is one link: http://library.thinkquest.org/10429/gather/jokes.htm Q. What is the dieter's element? A. Nobelium • Poem ...
08 PowerPoint
... hydrocarbon is hard to tell, but is usually a liquid after C=6 or higher. Most other covalent compounds are gases. Acids (chemicals starting with hydrogen) are always aqueous ...
... hydrocarbon is hard to tell, but is usually a liquid after C=6 or higher. Most other covalent compounds are gases. Acids (chemicals starting with hydrogen) are always aqueous ...
atom a very small particle that makes up most kinds of matters and
... two or more atoms of the same element that have different numbers of neutrons in the nuclei (same number of protons) states that mass is neither created nor destroyed and as a result the mass of the substances before a physical or chemical change is equal to the mass of the substances present after ...
... two or more atoms of the same element that have different numbers of neutrons in the nuclei (same number of protons) states that mass is neither created nor destroyed and as a result the mass of the substances before a physical or chemical change is equal to the mass of the substances present after ...
Chapter 3 - Warren County Schools
... broken down by physical or chemical means. – An atom of any element always has the same number of protons. ...
... broken down by physical or chemical means. – An atom of any element always has the same number of protons. ...
chemical bonds - geraldinescience
... • The forces that hold together the atoms in molecules are called chemical bonds. • Chemical bonds form because of the attraction between positive and negative charges. • Atoms form chemical bonds by either sharing or transferring electrons from one atom to another. • Scientists can study interactio ...
... • The forces that hold together the atoms in molecules are called chemical bonds. • Chemical bonds form because of the attraction between positive and negative charges. • Atoms form chemical bonds by either sharing or transferring electrons from one atom to another. • Scientists can study interactio ...
Chemical reactions
... Percent Composition of Compounds • Finding the mass percentage of an individual element from the formula weight ...
... Percent Composition of Compounds • Finding the mass percentage of an individual element from the formula weight ...
CH 11 Chemical Reaction WS #2 (Pre
... CH 11 Chemical Reaction WS #2 (Pre-lab) Name_____________________________ 1. What is the Great Barrier Reef and how was it formed? 2. Define chemical reaction3. How is a chemical reaction different from a physical one? Provide examples to support your explanation. 4. Explain how the appearance of th ...
... CH 11 Chemical Reaction WS #2 (Pre-lab) Name_____________________________ 1. What is the Great Barrier Reef and how was it formed? 2. Define chemical reaction3. How is a chemical reaction different from a physical one? Provide examples to support your explanation. 4. Explain how the appearance of th ...
IB1 Introduction to Ch
... other substances Depend on the amount of material Independent of the amount of material ...
... other substances Depend on the amount of material Independent of the amount of material ...
High School Chemistry Essential Questions
... 2. What observations about chemical systems and chemical interactions lead us to form the physical, graphical, and mathematical models that we use to represent, analyze, and communicate structure and relationships in chemical systems and chemical interactions? 3. How do we use the physical models, s ...
... 2. What observations about chemical systems and chemical interactions lead us to form the physical, graphical, and mathematical models that we use to represent, analyze, and communicate structure and relationships in chemical systems and chemical interactions? 3. How do we use the physical models, s ...
Chemistry lesson note
... APPLICATION OF CHEMISTRY • FOOD:- Chemistry is used to increase food production by the use of fertilizer and insecticides, preservation and addition of essential nutrients to improve the quality of food • CLOTHING:- Textile fibres are produced by chemical research • HOUSING:- Cement, concretes, bri ...
... APPLICATION OF CHEMISTRY • FOOD:- Chemistry is used to increase food production by the use of fertilizer and insecticides, preservation and addition of essential nutrients to improve the quality of food • CLOTHING:- Textile fibres are produced by chemical research • HOUSING:- Cement, concretes, bri ...
Chemical Reactions
... can only change forms So when we write equations… The number of each type of atom on the reactants side must be equal to the number of each type of atom on the products side ...
... can only change forms So when we write equations… The number of each type of atom on the reactants side must be equal to the number of each type of atom on the products side ...
Simple Chemical Reactions
... 40 cm3 Industrial denatured alcohol (IDA is highly flammable) If you are planning on using alternative fuels contact SSERC first for advice. ...
... 40 cm3 Industrial denatured alcohol (IDA is highly flammable) If you are planning on using alternative fuels contact SSERC first for advice. ...
Document
... 2HCl(aq) + Cr(s) H2(g)+ CrCl2(aq) A. composition B. single-displacement C. decomposition D. double-displacement ...
... 2HCl(aq) + Cr(s) H2(g)+ CrCl2(aq) A. composition B. single-displacement C. decomposition D. double-displacement ...
Unit 1 Matter Day 32 2016 Counting Atoms
... Def. This law states that matter(mass) CANNOT be created or destroyed in ordinary physical or chemical changes. The total mass of the reactants equals the total mass of the products. This can be proven in a closed system… one in which nothing enters or leaves the ...
... Def. This law states that matter(mass) CANNOT be created or destroyed in ordinary physical or chemical changes. The total mass of the reactants equals the total mass of the products. This can be proven in a closed system… one in which nothing enters or leaves the ...
Chemistry Notes
... Separate the water in salt water from the salts Boil off the water and salts will remain Separate a mixture of gases Cool them – they will condense at different temperatures ...
... Separate the water in salt water from the salts Boil off the water and salts will remain Separate a mixture of gases Cool them – they will condense at different temperatures ...
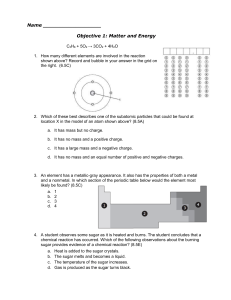
Name Objective 1: Matter and Energy C3H8 + 5O2 → 3CO2 + 4H2O
... 16. Which two compounds contain the same total number of atoms? (8.5D) a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. fo ...
... 16. Which two compounds contain the same total number of atoms? (8.5D) a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. fo ...
Chemical Corps

The Chemical Corps is the branch of the United States Army tasked with defending against chemical, biological, radiological, and nuclear (CBRN) weapons. The corps was founded as the Chemical Warfare Service (CWS) during World War I. Its name was changed to the Chemical Corps in 1946.