Writing and Classifying Balanced Equations
... Write the word or words that best completes each sentence. balanced, product, separation, reactants, chemical reaction, or element ...
... Write the word or words that best completes each sentence. balanced, product, separation, reactants, chemical reaction, or element ...
chemical warfare world war i
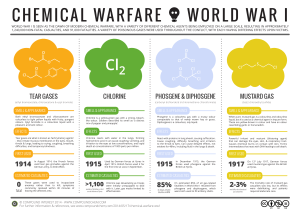
... In August 1914, the French forces used tear gas grenades against the German army, to little effect. ...
... In August 1914, the French forces used tear gas grenades against the German army, to little effect. ...
Outline Chapter 10 The Periodic Law
... 10-13. Ionic Bond Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a metal atom combines with a nonmetal atom to form an ionic compound, the chemical formula of the ionic compound fo ...
... 10-13. Ionic Bond Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a metal atom combines with a nonmetal atom to form an ionic compound, the chemical formula of the ionic compound fo ...
Document
... In a chemical equation, like the one below, you will notice that there are regular sized numbers in front of some of the molecules and small numbers after certain atoms within a molecule. The little number is called the subscript and tells how many of a certain type of atom are in a molecule. The bi ...
... In a chemical equation, like the one below, you will notice that there are regular sized numbers in front of some of the molecules and small numbers after certain atoms within a molecule. The little number is called the subscript and tells how many of a certain type of atom are in a molecule. The bi ...
Class Activity
... Chemical Change, Symbols, and Separation of Mixtures Physical change: A change in the state of matter. It does not result in a new type of substance. For example, melting wax or ice. Most of the physical changes are reversible (you can change them back easily). Physical properties are associated wit ...
... Chemical Change, Symbols, and Separation of Mixtures Physical change: A change in the state of matter. It does not result in a new type of substance. For example, melting wax or ice. Most of the physical changes are reversible (you can change them back easily). Physical properties are associated wit ...
Ch. 6: Chemical Reactions Study Guide
... In endothermic reactions energy is transferred from the surroundings into the reactants. An endothermic reaction is one in which heat is transferred from the surroundings to the reactants. In an exothermic reaction, energy is transferred from the reactants to the surroundings. A chemical reaction th ...
... In endothermic reactions energy is transferred from the surroundings into the reactants. An endothermic reaction is one in which heat is transferred from the surroundings to the reactants. In an exothermic reaction, energy is transferred from the reactants to the surroundings. A chemical reaction th ...
File - Flipped Out Science with Mrs. Thomas!
... 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing substances 8.5F recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of ...
... 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing substances 8.5F recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of ...
Classification of Matter
... Elements & Symbols • The symbol of an element is often taken from its name. • The first letter is always capitalized. • If an element starts with the same letter as another element, sometime the first two letters are used. • The second letter is always lowercase. • Some elements have symbols that d ...
... Elements & Symbols • The symbol of an element is often taken from its name. • The first letter is always capitalized. • If an element starts with the same letter as another element, sometime the first two letters are used. • The second letter is always lowercase. • Some elements have symbols that d ...
Chemistry: Introduction to Chemical Reactions Guided Inquiry What
... for calcium oxide, the ions are Ca2+ and 02-, the subscripts are Ca2O2 which should be simplified to CaO; for aluminum sulfate, the ions are Al3+ and SO42- , the subscripts are Al2(SO4)3 . c. For molecular compounds, combine the elements so each element has a full valence shell of electrons (satisfi ...
... for calcium oxide, the ions are Ca2+ and 02-, the subscripts are Ca2O2 which should be simplified to CaO; for aluminum sulfate, the ions are Al3+ and SO42- , the subscripts are Al2(SO4)3 . c. For molecular compounds, combine the elements so each element has a full valence shell of electrons (satisfi ...
Core Idea PS1 Matter and Its Interactions How can one explain the
... periods (orders elements horizontally by the number of protons in the atom’s nucleus) families (place those with similar chemical properties in columns) valence (reflect patterns of outer electron states) ...
... periods (orders elements horizontally by the number of protons in the atom’s nucleus) families (place those with similar chemical properties in columns) valence (reflect patterns of outer electron states) ...
Chemical Synthesis (sat6)
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
Experiment #5 WHERE`S THE EVIDENCE
... Matter is anything that has mass and takes up space. The study of matter and how matter changes is called chemistry. Matter can be described in terms of two kinds of properties—physical properties and chemical properties. Changes in matter can be described in terms of physical changes and chemical c ...
... Matter is anything that has mass and takes up space. The study of matter and how matter changes is called chemistry. Matter can be described in terms of two kinds of properties—physical properties and chemical properties. Changes in matter can be described in terms of physical changes and chemical c ...
Matter
... In a system of two or more phases, each phase is a visibly different part which has different properties The variation in properties may be : - different physical properties in each phase - different chemical properties - different physical and chemical properties ...
... In a system of two or more phases, each phase is a visibly different part which has different properties The variation in properties may be : - different physical properties in each phase - different chemical properties - different physical and chemical properties ...
Unit 2.2 Test Review Key
... 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing substances 8.5F recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of ...
... 8.5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing substances 8.5F recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of ...
pretest - Allen County Schools
... 11. When you have matter change in properties only what kind of change do you have? a. physical c. no change b. chemical d. both chemical and physical 12. When you have matter change in color AND composition of matter what kind of change do you have? a. physical c. no change b. chemical d. both chem ...
... 11. When you have matter change in properties only what kind of change do you have? a. physical c. no change b. chemical d. both chemical and physical 12. When you have matter change in color AND composition of matter what kind of change do you have? a. physical c. no change b. chemical d. both chem ...
ppt
... total mass of the reactants is equal to the total mass of the products. Atoms are not created nor destroyed. ...
... total mass of the reactants is equal to the total mass of the products. Atoms are not created nor destroyed. ...
CHEMICAL REACTIONS
... (g) after the formula –gas H2 (g) (l) after the formula -liquid H2O(l) (aq) after the formula - dissolved in water, an aqueous solution. CaCl2 (aq) used after a product indicates a gas (same as (g)) O2 used after a product indicates a solid (same as (s)) CaCo3 ...
... (g) after the formula –gas H2 (g) (l) after the formula -liquid H2O(l) (aq) after the formula - dissolved in water, an aqueous solution. CaCl2 (aq) used after a product indicates a gas (same as (g)) O2 used after a product indicates a solid (same as (s)) CaCo3 ...
Activity 14: Physical and Chemical Properties of Materials
... • A property is a quality or trait that characterizes a material or object. • Physical Properties can be determined without a chemical reaction. • Chemical Properties can only be determined by looking for a reaction. • Chemical Reaction is when a substance changes chemically into another substance. ...
... • A property is a quality or trait that characterizes a material or object. • Physical Properties can be determined without a chemical reaction. • Chemical Properties can only be determined by looking for a reaction. • Chemical Reaction is when a substance changes chemically into another substance. ...
Chemical Equations and Reaction Types Lab
... equation for a reaction cannot be written unless the substances that are reacting and being formed are both ...
... equation for a reaction cannot be written unless the substances that are reacting and being formed are both ...
AP Chemistry Ch. 3 Sections 3.7-3.8 Notes Chemical Equations
... Ch. 3 Sections 3.7-3.8 Notes Chemical Equations, Balancing Chemical Equations Chemical Equations • Chemical change – reorganization of the atoms in one or more substances. • Represented by a chemical equation with the reactants on the left side of an arrow and the products on the right side. • CH4 + ...
... Ch. 3 Sections 3.7-3.8 Notes Chemical Equations, Balancing Chemical Equations Chemical Equations • Chemical change – reorganization of the atoms in one or more substances. • Represented by a chemical equation with the reactants on the left side of an arrow and the products on the right side. • CH4 + ...
Test
... color, and shape. c. They explain how the substance reacts with other things d. They describe what chemical changes the substance is currently going through. While investigating a new substance, Megan and Tyler recorded the following observations: The new substance is solid. The new substance forms ...
... color, and shape. c. They explain how the substance reacts with other things d. They describe what chemical changes the substance is currently going through. While investigating a new substance, Megan and Tyler recorded the following observations: The new substance is solid. The new substance forms ...
Balancing Equations
... • I can list the main types of chemical reactions. • I can identify reactants and products in a chemical equation. • I can balance chemical equations when all reactants and products are given. • I can classify reactions by major type and can predict products of simple reactions. • I can apply the st ...
... • I can list the main types of chemical reactions. • I can identify reactants and products in a chemical equation. • I can balance chemical equations when all reactants and products are given. • I can classify reactions by major type and can predict products of simple reactions. • I can apply the st ...
Chemical Corps

The Chemical Corps is the branch of the United States Army tasked with defending against chemical, biological, radiological, and nuclear (CBRN) weapons. The corps was founded as the Chemical Warfare Service (CWS) during World War I. Its name was changed to the Chemical Corps in 1946.