Matter - Clayton State University
... - A combination of two or more pure substances Examples grains of rice and wheat cereal and sugar salt and sand - Components of a mixture can be separated by physical means (filtration, distillation, the use of magnet for metals) ...
... - A combination of two or more pure substances Examples grains of rice and wheat cereal and sugar salt and sand - Components of a mixture can be separated by physical means (filtration, distillation, the use of magnet for metals) ...
weekly schedule and topics
... This course will discuss the fundamental issues and problems related to a range of topics which are currently at the forefront of heavy inorganic industrial chemistry. The general topics deal with such areas as the development of industrial chemical processes, the environmental protection and air po ...
... This course will discuss the fundamental issues and problems related to a range of topics which are currently at the forefront of heavy inorganic industrial chemistry. The general topics deal with such areas as the development of industrial chemical processes, the environmental protection and air po ...
Chemistry Note PowerPoint
... Valance Electrons and Bonding • An atom’s valance electrons are those that have the highest energy levels and are held most loosely. • The number of valance electrons determine many properties of that element, including the ways in which the atom combines with other atoms ...
... Valance Electrons and Bonding • An atom’s valance electrons are those that have the highest energy levels and are held most loosely. • The number of valance electrons determine many properties of that element, including the ways in which the atom combines with other atoms ...
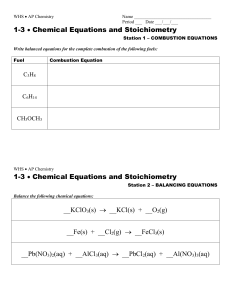
Balancing Chemical Equations
... 3. You can only add coefficients a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
... 3. You can only add coefficients a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
Describing Chemical Reactions
... written on the left side of the equation, followed by an arrow ( ). You read the arrow as “yields.” The formulas for the products are written on the right side of the equation. When there are two or more reactants or products, they are separated by plus signs. The principle called conservation of ma ...
... written on the left side of the equation, followed by an arrow ( ). You read the arrow as “yields.” The formulas for the products are written on the right side of the equation. When there are two or more reactants or products, they are separated by plus signs. The principle called conservation of ma ...
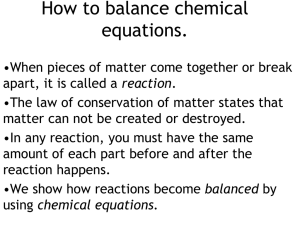
How to balance chemical equations.
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
COUNTING ATOMS
... • Coefficients are numbers that appear before elements in a chemical equation that change the number of reactants or products. • Example: • H2 + O2 H20 • The correct way to write this equation is: • 2H2 + O2 2H2O • The coefficients change the number of hydrogen and water molecules present. ...
... • Coefficients are numbers that appear before elements in a chemical equation that change the number of reactants or products. • Example: • H2 + O2 H20 • The correct way to write this equation is: • 2H2 + O2 2H2O • The coefficients change the number of hydrogen and water molecules present. ...
Physical Science CP Seton Hall Preparatory School Mr. Greene
... Mass (scientific definition) plus units (i.e. g, kg) Gravity (scientific definition) Temperature (scientific definition) plus units and conversions Density (scientific definition) plus density calculation (know formula) Area calculation and units Volume calculation and units Conversion factors and t ...
... Mass (scientific definition) plus units (i.e. g, kg) Gravity (scientific definition) Temperature (scientific definition) plus units and conversions Density (scientific definition) plus density calculation (know formula) Area calculation and units Volume calculation and units Conversion factors and t ...
Science 9
... Phase describes a PHYSICAL state of matter. Matter “moves” from one phase to another by physical forces such as temperature and pressure. If energy is added (e.g., increased temperature) or taken away (e.g., freezing), a physical change is created. SOLID + e = LIQUID + e = GAS + e = PLASMA Any kind ...
... Phase describes a PHYSICAL state of matter. Matter “moves” from one phase to another by physical forces such as temperature and pressure. If energy is added (e.g., increased temperature) or taken away (e.g., freezing), a physical change is created. SOLID + e = LIQUID + e = GAS + e = PLASMA Any kind ...
physics/0010052 PDF
... chemical processes measured by calorimetry and by the Van’t-Hoff equation differs very much from each other. The result is confirmed by many experiments. ...
... chemical processes measured by calorimetry and by the Van’t-Hoff equation differs very much from each other. The result is confirmed by many experiments. ...
21:3 Classifying Chemical Reactions
... called fungi. There are many kinds of yeasts, some of them of great importance to humans. Yeast is necessary to make leavened bread, beer, and cheese. It is rich in B vitamins; a form of yeast called brewer's yeast is used as a diet supplement. Yeasts are found in the soil, in water, on the surface ...
... called fungi. There are many kinds of yeasts, some of them of great importance to humans. Yeast is necessary to make leavened bread, beer, and cheese. It is rich in B vitamins; a form of yeast called brewer's yeast is used as a diet supplement. Yeasts are found in the soil, in water, on the surface ...
Semester 1 Final Review Powerpoint
... (they do not have to be equal in number). • The nuclear components are held together by the nuclear strong force. ...
... (they do not have to be equal in number). • The nuclear components are held together by the nuclear strong force. ...
Honors Chapter 2
... Matter can be a gas, a liquid, or a solid. Gases have no fixed shape or volume. Gases can be compressed to form liquids. Liquids have no shape, but they do have a volume. Solids are rigid and have a definite shape and volume. ...
... Matter can be a gas, a liquid, or a solid. Gases have no fixed shape or volume. Gases can be compressed to form liquids. Liquids have no shape, but they do have a volume. Solids are rigid and have a definite shape and volume. ...
Describing Matter Chapter 2:2 Physical and Chemical Properties
... easy to undo • Chemical Properties~ a property of matter that describes a substance based on its ability to change into a new substance with different properties • Chemical Change~ a change that occurs when one or more substances are changed into entirely new substances with different properties; ca ...
... easy to undo • Chemical Properties~ a property of matter that describes a substance based on its ability to change into a new substance with different properties • Chemical Change~ a change that occurs when one or more substances are changed into entirely new substances with different properties; ca ...
Chapter One Powerpoint - Geneva Area City Schools
... • solid state, matter has definite volume and definite shape. • liquid state, matter has a definite volume but an indefinite shape. • gas state, matter has neither definite volume nor definite shape. • Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, ...
... • solid state, matter has definite volume and definite shape. • liquid state, matter has a definite volume but an indefinite shape. • gas state, matter has neither definite volume nor definite shape. • Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, ...
Student Expectation
... Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are called “valence electrons” and h ...
... Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are called “valence electrons” and h ...
Introduction to Chemistry and Measurement
... close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun. ...
... close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun. ...
Chemical Properties - Michigan State University
... what is occurring in the lab. How has the sugar changed? (asked after the physical AND chemical change) Is the sugar still present? How are the physical and chemical changes different? How would you classify a physical change? What about a chemical change? I want to discuss and ask a question also a ...
... what is occurring in the lab. How has the sugar changed? (asked after the physical AND chemical change) Is the sugar still present? How are the physical and chemical changes different? How would you classify a physical change? What about a chemical change? I want to discuss and ask a question also a ...
[Mg] +2[ S ]-2
... 11. Adding ice cubes to hot chocolate so it cools down faster not a chemical reaction 12. The smell that is given off from a stink bomb chemical reaction Using the 5 indicators of chemical reactions explain how you can determine whether a chemical reaction has taken place or not in the scenario belo ...
... 11. Adding ice cubes to hot chocolate so it cools down faster not a chemical reaction 12. The smell that is given off from a stink bomb chemical reaction Using the 5 indicators of chemical reactions explain how you can determine whether a chemical reaction has taken place or not in the scenario belo ...
Honors Midterm - Stamford High School
... 3. Make an element inventory. How are you going to know if the equation is balanced if you don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole prob ...
... 3. Make an element inventory. How are you going to know if the equation is balanced if you don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole prob ...
Ch. 3 9-Station Review
... When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
... When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
Chemical equations must be balanced.
... has a subscript of 2, which means there are two hydrogen atoms. Also, there are two oxygen atoms on the left and three oxygen atoms on the right. Because of the conservation of mass, you know that hydrogen atoms do not disappear and oxygen atoms do not suddenly appear. ...
... has a subscript of 2, which means there are two hydrogen atoms. Also, there are two oxygen atoms on the left and three oxygen atoms on the right. Because of the conservation of mass, you know that hydrogen atoms do not disappear and oxygen atoms do not suddenly appear. ...
Lecture Notes
... which a substance changes from the solid state to the liquid state. For pure water themelting point is 32oF or 0oC. The Freezing point of a substance is the same as the melting point, since the process of freezing is the opposite of melting. The boiling point is defined as that temperature at which ...
... which a substance changes from the solid state to the liquid state. For pure water themelting point is 32oF or 0oC. The Freezing point of a substance is the same as the melting point, since the process of freezing is the opposite of melting. The boiling point is defined as that temperature at which ...
Chemical Corps

The Chemical Corps is the branch of the United States Army tasked with defending against chemical, biological, radiological, and nuclear (CBRN) weapons. The corps was founded as the Chemical Warfare Service (CWS) during World War I. Its name was changed to the Chemical Corps in 1946.


















![[Mg] +2[ S ]-2](http://s1.studyres.com/store/data/014450548_1-468f3af464a09baae245d79fadf97d41-300x300.png)